GxP Lifeline
Top 5 Artificial Intelligence Tools for Quality and Manufacturing Professionals
 The recent explosion of artificial intelligence (AI) applications opens a world of possibility in the life sciences industry, offering the potential for tools integrated across quality and manufacturing operations that can streamline processes, enhance efficiency, and ensure best-in-class quality standards. But professionals working in life sciences companies need more than hype – they need practical uses for powerful new tools. That’s why they’re continually asking questions like, “How can we use AI for quality control in manufacturing?” or “How can I apply AI in quality management?"
The recent explosion of artificial intelligence (AI) applications opens a world of possibility in the life sciences industry, offering the potential for tools integrated across quality and manufacturing operations that can streamline processes, enhance efficiency, and ensure best-in-class quality standards. But professionals working in life sciences companies need more than hype – they need practical uses for powerful new tools. That’s why they’re continually asking questions like, “How can we use AI for quality control in manufacturing?” or “How can I apply AI in quality management?"
To better understand the role of AI in life sciences manufacturing and to learn which specific AI tools would be the most beneficial to their operations, MasterControl recently conducted a research study with over 100 quality and manufacturing professionals in the life sciences. These respondents see huge potential, and make it clear the benefits they see to their process improvements far outweigh concerns about using AI in their roles.
Top 3 Benefits for Quality and Manufacturing Professionals in Their Day-to-Day Role
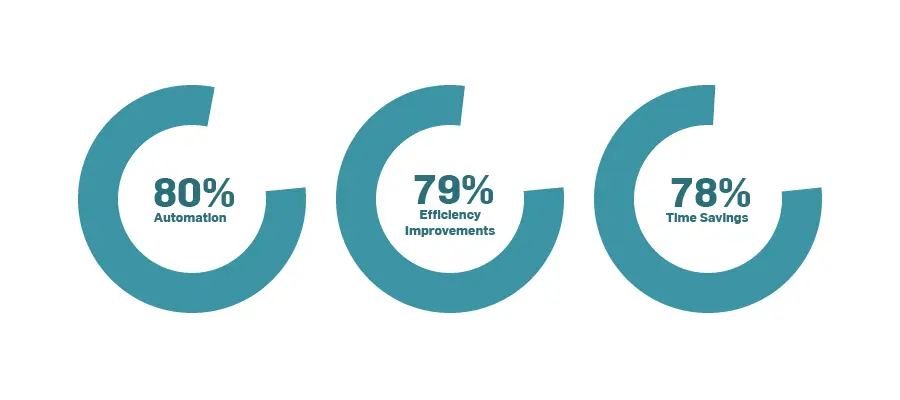
Automation, efficiency improvements, and time savings are tightly interrelated. Our research
identified several use cases where this triad brought value. Those included an exam generator that automates the exam creation process to improve efficiency and save time and does a better job at ensuring training comprehension than a traditional “read-and-understood” exam can do. A document translator was also identified as valuable because automated translations can speed the translation process and create production efficiencies.
The top five artificial intelligence tools identified by quality and manufacturing professionals are outlined below. Each tool expresses the three primary benefits of AI in quality management and life sciences manufacturing.
1. Automated Risk Assessment
What It Does: Automates data collection, predictive analytics, compliance risk, and more to recognise and categorise the severity of a quality event. Leverages a large language model (LLM) to analyse historical data, identify patterns and trends, and more to calculate a risk score for an individual event.

Benefits: By identifying risk score, quality managers can prioritise events based on risk, allowing them to focus on high-impact issues that need more resources for corrective action/preventive action (CAPA). This can save time, minimise the recurrence of events, and more.
2. KPI Analyzer
What It Does: Evaluates key performance indicators (KPIs) created in a quality quality management system (QMS)management system (QMS) (QMS) or electronic batch electronic batch record (EBR) (EBR), creates a summary of performance against benchmarks, and makes recommendations for recovery or improvement.

Benefits: By being able to automatically monitor KPIs, manufacturing and quality professionals can quickly identify opportunity areas and make data-driven decisions to optimise processes. This leads to enhanced productivity, better resource utilisation, and ultimately, higher-quality outputs.
3. APQR (Annual Product Quality Review) Generator
What It Does: An APQR generator utilises AI in quality management to automate the process of conducting required annual product quality reviews. It identifies key trends that can drive continuous improvement, in addition to producing a draft report for review.

Benefits: By automating the APQR process, manufacturers can save hundreds of hours of collecting and analysing system data and valuable resources while ensuring compliance with regulatory requirements. The resulting report can influence important decisions regarding product manufacturing, quality control, and more.
4. Auto Lot Tracking Report Generator
What It Does: Automatically runs a report to investigate a batch. Shows all the production records, open CAPA, management of changes (MOCs) in process, supplier issues, and more to help resolve issues and prepare for submissions to the U.S. Food and Drug Administration (FDA).

Benefits: By automating lot tracking reports, manufacturers can streamline compliance processes and enhance traceability, which is crucial for ensuring product safety and quality. This tool also enables quick identification and resolution of any issues or discrepancies, minimising the risk of recalls or quality incidents.
5. Quality Event Report Summary Generator
What It Does: Identifies and categorises quality events events and then generates a concise summary that highlights the most critical aspects of each quality event.

Benefits: By utilising an LLM for monthly quality event evaluation and reporting, AI can enhance quality management processes, reduce risks, and continually improve product quality and compliance.
Conclusion
AI-driven tools are poised to transform quality and manufacturing processes by assisting users to enhance efficiency, streamline processes, and improve quality standards. Implementation of these low-risk, highly valuable AI tools across internal quality and manufacturing processes can ultimately lead to thousands of hours in manual tasks saved, and millions of dollars that can be reallocated to R&D, continuous improvement, and other efforts.
