Executive Report: Digital Maturity in Medical Device Quality and Manufacturing
The innovative, breakthrough product advancements medical device manufacturers are introducing to the market continue to revolutionise health care as we know it.
Yet, as a manufacturer in this industry, you bear the burden of meeting the absolute highest quality and safety standards – without compromise. Not only are you required to design and develop products according to the highest quality and regulatory standards, but you must also ensure that Quality by Design (QbD) principles are implemented and maintained throughout long, complex product development, clinical, and regulatory life cycles. Plus, you must deliver flawless execution at every phase of the supply chain and manufacturing process.
Meeting these standards leaves no room – or time – for error or delay. Yet you still face daily challenges and more demanding production requirements that impede your ability to develop and deliver flawlessly manufacturing products. Your critical role in bringing breakthrough, life-changing products to patients in a highly regulated industry can often feel like an endless series of trade-offs.
Common Product Delivery Trade-Offs Life Sciences Medical Device Face
With the continued evolution of advanced technologies used in the development of medical devices today, they require more complex and connected processes for manufacturing. However, one key trade-off the industry continues to face is a lack of departmental alignment, leading to a disconnection across product development, manufacturing operations, quality management, supply chain, and more.
Organisational alignment and harmonisation across departments, suppliers, and systems can mean the difference between missed deliveries, stock outs, and product recalls versus operational excellence that will allow life-changing products to be delivered faster, more efficiently, and more affordably to patients and consumers. However, each department’s priorities, metrics, technologies, and databases too easily become disconnected and disjointed and are too often in competition with one another.
Medical Device Organisation Top Priorities For Quality and Manufacturing Departments
Manufacturing
- Decrease operational expenses and reduce waste.
- Increase data visibility to improve efficiencies in processes.
- Connect and integrate systems.
- Automate production workflows.
- Improve inventory management and tracking.
- Establish more efficient labor management.
Quality
- Reduce costs associated with quality issues during production.
- Improve data visibility and connectivity (e.g., connecting essential data across every point in the product life cycle).
- Automate production workflows.
- Pass audits and meet (or even exceed) regulatory requirements.
- Streamline workflows, particularly with document management and training tasks.
- Connect and integrate systems.
This isn’t a new dynamic, but as the cost, complexity, and velocity of bringing products to market has increased, it’s becoming increasingly important for medical device organisations to drive both departmental alignment and digital system integration and advancement to achieve their priorities. This requires a cross-functional commitment to and investment in digitalisation and technology that facilitates more connected and intelligent operations to aid in delivery of next gen medtech.
To maintain a competitive edge amidst increasing complexities in the industry today, medical device manufacturers must have digital maturity at the top of priority lists across the organisation. Through a recent research study conducted by MasterControl, medical device professionals confirm that they recognise the importance of digital transformation and digital maturity. They recognise how vital it is to fully implement, adopt, and optimise digital systems to reduce trade-offs, stay competitive in the market, and achieve organisational goals. However, there is a gap between where their digital maturity is today and where they aim to be – a gap that is causing them to make the trade-offs noted above that lead to inefficiencies and wasted time, effort, and resources.
This report is the culmination of MasterControl’s in-depth research aimed at understanding 1) how important digitalisation is to process improvement and 2) where medical device organisations stand on the digital maturity spectrum. It is based on survey responses from quality and manufacturing professionals from medical device companies of a wide range of sizes and annual revenues. Their feedback offers insight into the opportunities digital maturity presents, the challenges and realities preventing companies from fully realising their digital transformation potential, and how MasterControl can close gaps to help them achieve smarter processes, accelerate production, and deliver life-changing therapies sooner.
The Opportunity: Unifying Digital Maturity Imperatives
Our research found that both quality and manufacturing medtech professionals agree – their organisations need to prioritise digitalisation initiatives.
The data indicates they are often targeting the same or similar outcomes, yet departmental gaps may undercut the value of digital efforts if there isn’t a concerted effort to unify quality and manufacturing data, systems, and operations.
A majority of respondents agree: digitalisation is a high priority
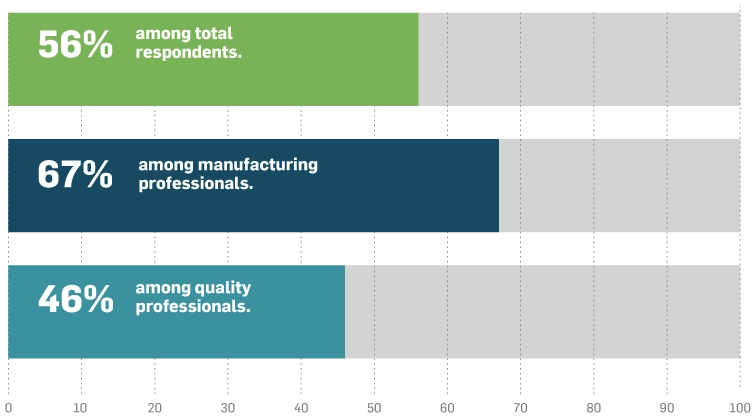
Among organisations that consider digitalisation a high priority, 65% of medical device executivest feel positively about increasing the level of digitalisation and technological advancement in their processes.
Digitalisation Benefits According to One Medical Device Executive:
In our opinion, increase use of advanced digital technology will be beneficial for automated data collection, inventory tracking, as well as improving production planning.
– Line Management Executive, Medical Device Manufacturer
Digitalisation doesn’t happen in a silo at the executive level – it’s a top-to-bottom organisational shift, department harmonisation, and product life cycle optimisation that everyone needs to unify around for it to be successful. Yet today’s medtech executives recognise that efforts to digitalise processes will always compete with other initiatives their organisations are also under pressure to deliver on.
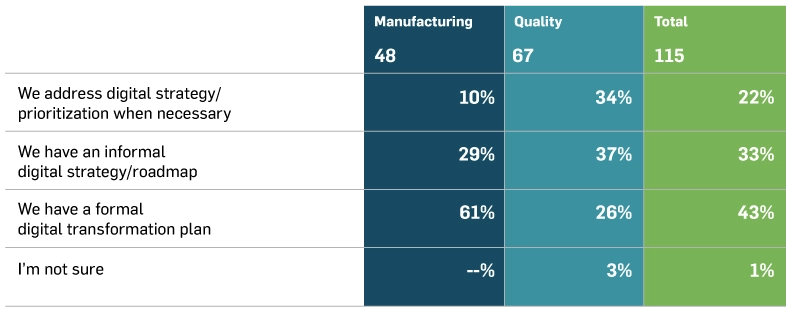
When asked about existing digital transformation initiatives, medical device organisations reported having varied plans in place:
Current Digital Transformation Plans for Medical Device Organisations
The Digital Maturity Spectrum
Just as every medical device manufacturer has unique business needs, they also have different degrees of digital maturity. To define and measure digital maturity for the study – and to help medtech companies see where they presently stand compared with where they want to be – we designed a digital maturity model with four tiers.
When shown this digital maturity model, respondents most commonly reported that their department is currently situated in the ‘Digital’ tier, while also recognising they would see significant benefits from maturing to more connected, more harmonised, more intelligent business systems supporting the product life cycle.
Manual
- Paper-based processes
- Offline data collection
Total
12%
Quality
11%
Manufacturing
12%

Digital
- Some digital systems/ processes in place
- In-line data capture
57%
60%
53%

Connected
- System integrations in place
- Automation
- Standardisation across sites
- Advanced analytics and data modeling
30%
26%
35%

Intelligent
- Paper-based processes
- Offline data collection
2%
3%
0%
Total
12%
Quality
11%
Manufacturing
12%
57%
60%
53%
30%
26%
35%
2%
3%
0%
Respondents were quick to point out the disconnected state of digitalisation that currently hinders manufacturing efforts.
"We have fully digital systems but they aren’t tied together but rather separate distinct systems," according to a Medical Device Organisation Site Manager.
"Our core processes are digitised, but they are not cloud-based," said one Senior Manager of QA for a Med Device Manufacturer.
"We have an electronic QMS...," noted a respondent working in a quality capacity.
There are significant organisational factors driving the orchestration of a focused effort on maturation, with ’Intelligent’ as the north star. The predominant theme across the industry is that digital maturity will allow medical device organisations to better compete, and more quickly adapt to change, under growing pressures to innovate while minimising trade-offs that could jeopardise safety or compliance.
Respondents' Top Five Motivations
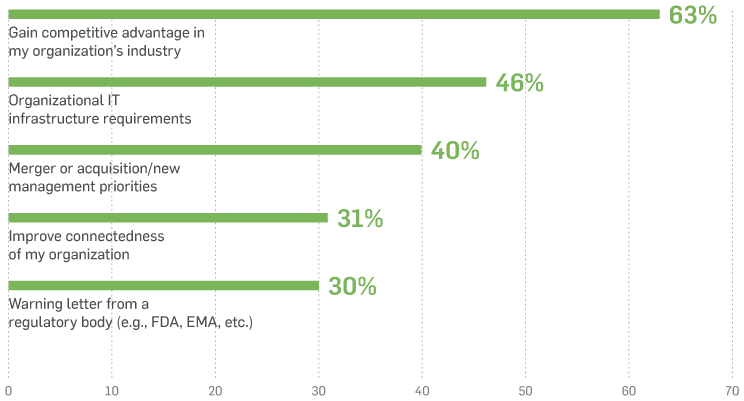
With even a modest increase in digital maturity, respondents recognise they’d be better prepared to:
- Anticipate disruptions.
- Move more quickly through approval cycles.
- Pass audits and inspections without issue or delay.
- Forecast for optimised batches.
- Predict and prevent overdue training.
These and many more benefits that come with enhanced digital maturity can help medical device manufacturers dramatically reduce errors and achieve more efficient production.
The Challenge: The Reality of Disjointed Digitalisation and Siloed Departments
Current processes are inefficient and time consuming. The lack of data visibility make it challenging to take action, and manual tasks are prone to errors that slow operations down. Current manual processes, siloed procedures, budget constraints, and data quality concerns within both manufacturing and quality departments continue to leave many pharma companies feeling limited in their ability to scale their operations. They're unable to truly realise the benefits of being connected.
Respondents' Pain Points With Current Processes
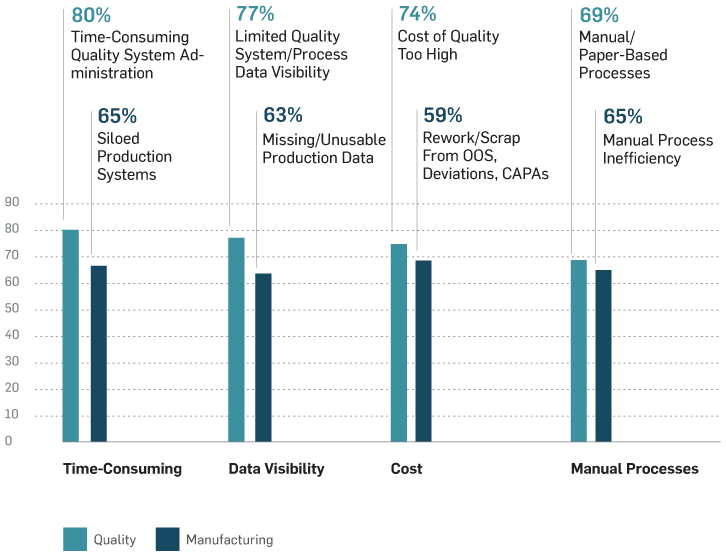
In general, medical device companies have been slow to truly adopt digital technologies. Their often paper-based, manual processes, time-consuming tasks, limited data visibility, and costs top their list of pain points and barrier preventing digital maturity.
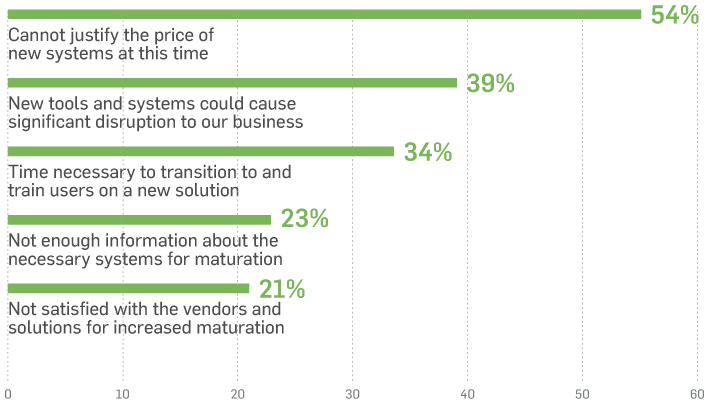
That means many medtech organisations today continue to live in a world of trade-offs. They’re making sacrifices and losing time or opportunities to innovate, and ultimately delaying product delivery to patients. The risk they perceive in implementing and adopting a new system to mature digitally and streamline processes can significantly outweigh the delays and costly mistakes experienced with their current manual processes.
Respondents' Top Five Barriers Preventing Increased Digital Maturity
As respondents were most likely to consider themselves ‘Digital,’ it’s not surprising that a majority of medical device organisations do report having purchased a quality management system (QMS) or manufacturing execution system (MES), , which on the surface, seems like positive momentum in their digital transformation journey. However, digging into the details of their implementations, it’s evident that companies are not seeing the full value of their investment in these systems today.
MasterControl’s research revealed that few have their digital systems fully implemented across all sites. Many are stuck in limbo with a mix of disconnected systems that can leave them feeling burdened with inefficiencies across their operations that are slowing down product delivery to patients depending on them.
Incomplete System Implementation Across Medical Device Companies
Manufacturing Challenges
More often than not, manufacturing batches still have some level of paper involved in production processes.
A majority of respondents (65%) reported that they already have an MES/digital manufacturing solution in place (N=48). However,
- Only 6% have an MES/digital manufacturing solution implemented at all facilities and sites.
- 66% have this technology fully implemented at some facilities.
- 28% are partially implemented.
Quality Challenges
Quality often has a mix of systems (Excel, SharePoint, QMS, etc.) for document workflows.
Most of respondents (94%) report they have a QMS at their facility (N=67). However,
- Only 42% have their QMS fully implemented at all facilities.
- 24% have this technology implemented at some facilities.
- 24% have only partially implemented their QMS.
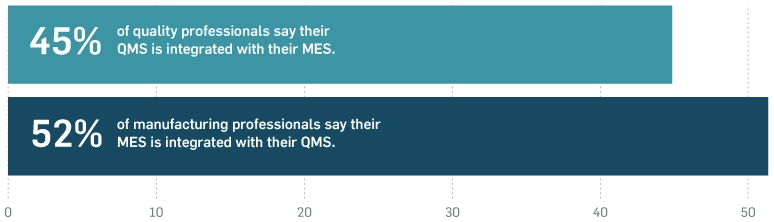
In addition to a lack of true system implementation is a lack of connectivity between the MES and QMS solutions that are in place. These nonexistent connections represent critical missing links between these departments that are preventing them from sharing workflows, insights, and more.
Many surveyed acknowledge the benefits of connected systems, yet they have not been able to align to make unified, connected operations a reality for their departments or the broader organisation.
The State of QMS/MES Connectivity
Cooperation: The Key to Overcoming Misperceptions
The perception that introducing a new system can cost too much or disrupt operations shouldn’t stop medical device organisations organisations from taking steps to achieve digital transformation. It doesn’t have to be a daunting undertaking and it doesn’t all have to be done at once. Taking an iterative approach and having departments in lockstep along each step will be key to success with digital maturity. Quality and manufacturing teams need to work together to streamline digitalisation across their shared processes, shared workflows, shared data visibility, shared training platform for compliance, and more.
Benefits of Digital Connectivity
Digitalisation aids in the collection of additional data and information for the purposes of improving the manufacturing process and gaining a better understanding of customer demands in the future. It facilitates in tracking the overall productivity and efficient supply flows.
– Director of Site Management, Medical Device Manufacturer
The Solution: The Connected MasterControl Platform
MasterControl allows you to take critical steps to deliver products without compromise, and without making trade-offs. We have more than 30 years of experience serving innovative companies in the medical device industry.
Our flexible QMS and modern EBR allows users to achieve ROI from their MasterControl investment in under a year by helping them avoid delayed deliveries and production bottlenecks, offsetting rising material prices with time and money saved automating, ultimately delivering innovations to patients faster.
- A purpose-built platform that allows you to scale across your organisation on your terms.
- Connected systems for seamless, cross-functional collaboration.
- A system that’s easy to implement and deploy.
- Low/no-code software for easy configuration.
- Native and third-party integrations to easily connect to other critical usiness systems.
- Risk-based, patented validation.
- Cross-platform, AI-powered analytics and insights.
With a robust connected system, the trade-offs you're used to making disappear, as do the gaps between departmental objectives and data. Enhanced and intelligent connectivity eliminates the need to decide whether safety is more important than speed because you can enjoy both. Most importantly, rather than forcing you to put quality in jeopardy for the sake of keeping costs low, an integrated system empowers you to achieve both by aligning product development, quality management, manufacturing operations, and the supply chain.
Common Trade-Offs
With Unconnected Systems

No Trade-Offs
With Connected Systems
MasterControl Platform
MasterControl’s validated platform allows for truly integrated processes to speed up time to market without sacrificing quality.
MasterControl
Manufacturing Excellence
Offers the fastest, most cost-effective path to 100% paperless manufacturing.
Features:
- Electronic Device History Records
- Equipment Calibration
- Equipment Maintenance
- Recipe Management
- Review by Exception
- Data Analytics and Insights
MasterControl
Quality Excellence
Helps over 300 medical device around the world make better decisions faster and accelerate their products’ time to market.
Features:
- Document Management
- Change Control
- Training and Exams
- Quality Events and CAPA
- Compliance and Audit Management
- Risk Management
- Data Analytics and Insights

What Customers Are Saying About MasterControl
Pharmaceutical leaders across the globe are seeing big results from their implementations of MasterControl. Here’s what they have to say:
Error Reduction
Today, we use the EBR functionality in MasterControl Manufacturing Excellence (Mx) which makes it easy for us to enter data and track all the information and actions completed in the system. All the information we need from our equipment is automatically logged in real time, meaning there are no data integrity issues, and we save time for the review of our batch records. That’s flexibility we didn’t have before.
– Enterprise Medical Device Manufacturer, EMEA
Enhanced Document Control
In a paper-based or hybrid paper/electronic system, it took us over a month to collaborate, create, and approve just one standard operating procedure, policy, or work instruction. With a centralised QMS, we are creating, revising, and approving on average nearly 30 core quality documents a month.
- Matthew Seitz-Paquette, Fagron North America Quality Specialist