
GxP Lifeline
Building a Connected Quality and Manufacturing Ecosystem in Life Sciences
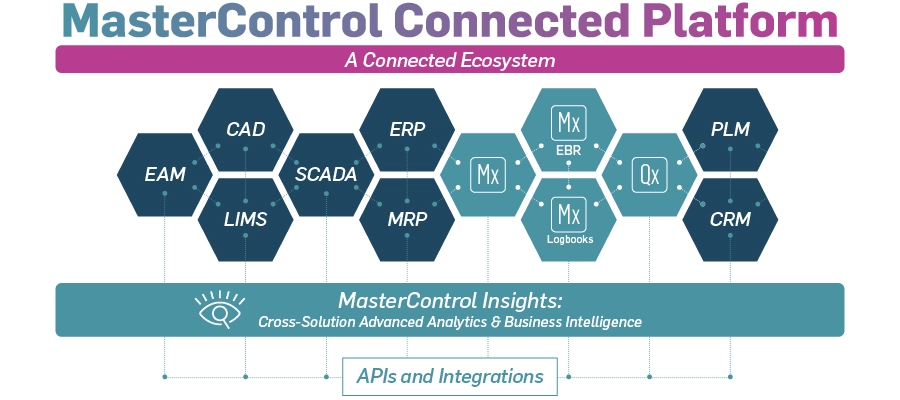
In today's rapidly evolving life sciences landscape, digital transformation in manufacturing is no longer a luxury – it's a necessity. As industry leaders seek to optimize operations, reduce costs, and improve quality, the concept of connected manufacturing has emerged as a game-changer. Recently, MasterControl hosted a webinar titled "Break Down Silos, Build a Connected Quality and Manufacturing Ecosystem," exploring how life sciences companies can leverage technology to create a more integrated, efficient, and compliant manufacturing environment.
For those who missed this insightful session, let's dive into some key takeaways from the Q&A portion, highlighting the importance of connected quality management systems (QMS) and manufacturing execution systems (MES) for life sciences.
Starting Small: The First Step Toward Digital Transformation
One of the primary concerns for many organizations is how to begin their digital transformation journey. The experts at MasterControl emphasize the importance of starting small and scaling up. This approach allows companies to see immediate benefits while minimizing disruption to existing processes.
When implementing a connected QMS or transitioning to paperless manufacturing, it's crucial to select the right technology partner. Look for providers who understand the unique challenges of life sciences manufacturing and can grow with your organization. As one MasterControl representative noted, "It's about finding a partner that's with you today and can stick with you into the future as you grow inside of your digital maturity."
Curious about how to kickstart your digital transformation? Watch the full webinar to gain more insights on building a connected ecosystem.
Real-World Results: The Power of Connected Systems
The webinar highlighted a compelling case study featuring Dendreon, a biopharmaceutical cellular therapy company that successfully implemented MasterControl's connected solutions. By digitizing and connecting their systems, Dendreon achieved remarkable results:
- 99% right-first-time metrics.
- 50% reduction in review and release times.
- Improved resource utilization, allowing them to treat more patients with existing resources.
These outcomes underscore the tangible benefits of integrated quality management for life sciences. By connecting manufacturing execution systems with quality management processes, companies can significantly enhance their operational efficiency and compliance.
To learn more about how other life sciences companies are benefiting from connected manufacturing, don't miss the full webinar recording.
Integrating New Systems With Existing Infrastructure
For many organizations, the challenge lies in integrating new digital solutions with existing systems. The MasterControl team addressed this concern, emphasizing the importance of cloud-based solutions and robust integration strategies.
When implementing electronic batch records (EBR) or other digital tools, it's crucial to consider how they will interact with your current quality management system. Look for solutions that offer seamless integration capabilities and can adapt to your specific needs.
"As you're looking for those EBR providers, just really be thoughtful about integration points," advised one of the MasterControl experts. "Can that provider grow with you?"
Discover more strategies for successful system integration by watching the complete webinar.
Overcoming Cost and Time Barriers
Cost and time constraints often pose significant barriers to digital transformation. However, the MasterControl team stressed the importance of focusing on the return on investment (ROI) that comes from automation and connected manufacturing.
For instance, seemingly small time savings, like reducing the time it takes to sign a batch record, can add up to hundreds of hours saved per year when multiplied across thousands of operations. Moreover, paperless manufacturing in life sciences can dramatically reduce errors, improving both efficiency and compliance.
"Focus on the real ROI that can come from automating and connecting these processes," emphasized one of the presenters. "It can span everything from things that take hours and become more efficient to things that take seconds and still have a huge impact on the bottom line."
To gain a deeper understanding of the ROI potential in connected manufacturing, be sure to watch the full webinar.
The Future of Connected Manufacturing in Life Sciences
As the life sciences industry continues to evolve, the trend towards fully integrated quality management and manufacturing execution systems is only going to accelerate. Companies that embrace digital transformation now will be better positioned to adapt to future challenges and opportunities.
Connected manufacturing isn't just about improving current processes – it's about creating a foundation for continuous improvement and innovation. By breaking down silos between quality, manufacturing, and other departments, life sciences companies can achieve unprecedented levels of efficiency, compliance, and product quality.
Conclusion: Embracing the Connected Future
The insights shared in MasterControl's webinar underscore the critical role of connected QMS and integrated manufacturing systems in advancing life sciences manufacturing. As the industry continues to face increasing regulatory pressures and market demands, the ability to seamlessly connect quality and manufacturing processes will become a key differentiator.
Whether you're just starting your digital transformation journey or looking to optimize your existing connected systems, the strategies and real-world examples discussed in this webinar provide valuable guidance.
Ready to take the next step in your digital transformation journey? Don't miss out on the wealth of information available in the full webinar. Watch "Break Down Silos, Build a Connected Quality and Manufacturing Ecosystem” now to learn how you can revolutionize your life sciences manufacturing processes with MasterControl's connected solutions.

