
GxP Lifeline
FDA Product Recall Avoidance: Your Guide to Preventing Regulatory Pitfalls
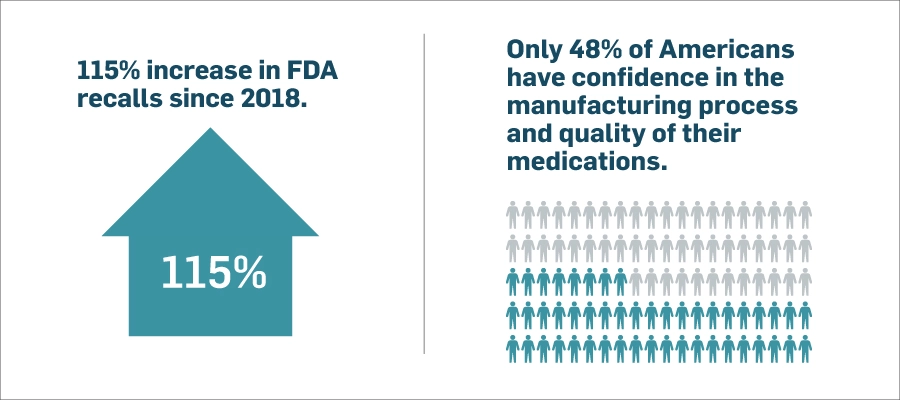
Receiving Form 483s or warning letters from the U.S. Food and Drug administration (FDA) and the potential for those observation issues to escalate to an FDA product recall are terrifying prospects for every life sciences company. And effectively managing product recall processes continues to pose significant challenges for companies throughout the industry. These challenges not only have financial implications but also can badly damage brand reputation and erode customer trust.
The Severity of Recall Issues
The number of FDA product recalls in the pharmaceutical and medical device industries has been steadily increasing in recent years. A recent MasterControl study revealed that the most common root causes of FDA product recalls include manufacturing defects, quality control issues, and noncompliance with good manufacturing practices (GMP). Additionally, receiving FDA Form 483 observations due to noncompliance with regulatory requirements can have serious consequences like delays in product approvals and potential enforcement actions.
The Solution: "Your Guide to FDA Product Recall Avoidance"
To address these challenges, MasterControl offers helpful tips in the "Your Guide to FDA Product Recall Avoidance" infographic. These recommendations are designed to help manufacturing and quality professionals in the life sciences navigate the intricate landscape of regulatory compliance and quality management. By leveraging this resource, organizations can proactively prevent product recalls, effectively manage potential issues, and mitigate the risk of receiving an FDA 483 warning letter.
Key Elements of Product Recall Management
The recommendations provided in the infographic are centered on five key components of product recall avoidance:
Comprehensive Regulatory Insights:
The infographic provides insights into the regulatory requirements that must be met to prevent FDA product recalls, highlighting key requirements and best practices for avoiding regulatory pitfalls.Risk Mitigation Strategies:
Practical strategies are provided for identifying and mitigating potential risks associated with product manufacturing, quality control, and compliance with GMP requirements.QMS Implementation Tactics:
The guide outlines actionable steps for implementing a robust quality management system (QMS) tailored to the unique needs of the life sciences industry. A purpose-built QMS simplifies adherence to regulatory standards and empowers organizations to foster a strong culture of quality and compliance.Proactive Recall Management Practices:
The infographic equips organizations with proactive recall management strategies, underscoring the importance of swift and effective responses to quality events in order to minimize the impact on patients and consumers.Compliance Training and Education:
The infographic emphasizes the importance of training and education in regulated environments. Comprehensive training programs instill a culture of compliance throughout the organization and reduce the likelihood of operational lapses that could lead to recalls or FDA enforcement actions.
The Path to FDA Product Recall Avoidance
The challenges posed by noncompliance and potential FDA product recalls necessitate a proactive and strategic approach to quality and compliance management in the life sciences industry. "Your Guide to FDA Product Recall Avoidance" offers invaluable insights and actionable steps to aid companies in their quest for regulatory excellence and product quality assurance.
By implementing the recommendations provided in "Your Guide to FDA Product Recall Avoidance," life sciences companies can proactively safeguard their operations, protect patient safety, and uphold their commitment to delivering high-quality products. It serves as a roadmap for establishing a culture of compliance, thereby reducing the risk of product recalls and safeguarding the organization's reputation and bottom line.
Take Action Today
Are you ready to fortify your organization against regulatory pitfalls and product recalls? Explore "Your Guide to FDA Product Recall Avoidance" and discover actionable insights and smart practices to mitigate risk, ensure compliance, and uphold the highest standards of product quality. Don't wait until a recall becomes a reality — be proactive and ensure your organization is equipped to navigate the complexities of regulatory compliance and quality management with confidence. Access this essential resource today to take the first step toward mastering FDA product recall avoidance.

