
GxP Lifeline
Validation 4.0: How AI Is Transforming Life Sciences Quality Management

Validation is undergoing a transformation. With the digital revolution in full swing, new technologies are emerging that have the potential to reshape the validation landscape. Amid the buzz surrounding artificial intelligence (AI) and machine learning (ML), the key question is: How can these cutting-edge tools enhance validation processes moving forward? More importantly, how can they drive the evolution toward Validation 4.0, improve life sciences quality management, and enable real-time process verification?
Understanding the Foundations of Validation 4.0
To understand the potential of Validation 4.0, it's essential to grasp its foundational principles. Validation 4.0 leverages the digitization and automation of pharmaceutical manufacturing (aligned with Pharma 4.0) to link real-time data with both the quality risk management system and control strategies. This connection enables continuous visibility into whether processes are in a state of control. A key component of this approach is mapping critical data, such as how critical process parameters (CPPs) are managed and where the data resides. For this model to function effectively, it must be built on strong data integrity and designed to ensure the data is accurate and reliable for making informed product quality decisions within the life sciences quality management system. (See Figure 1 below.)
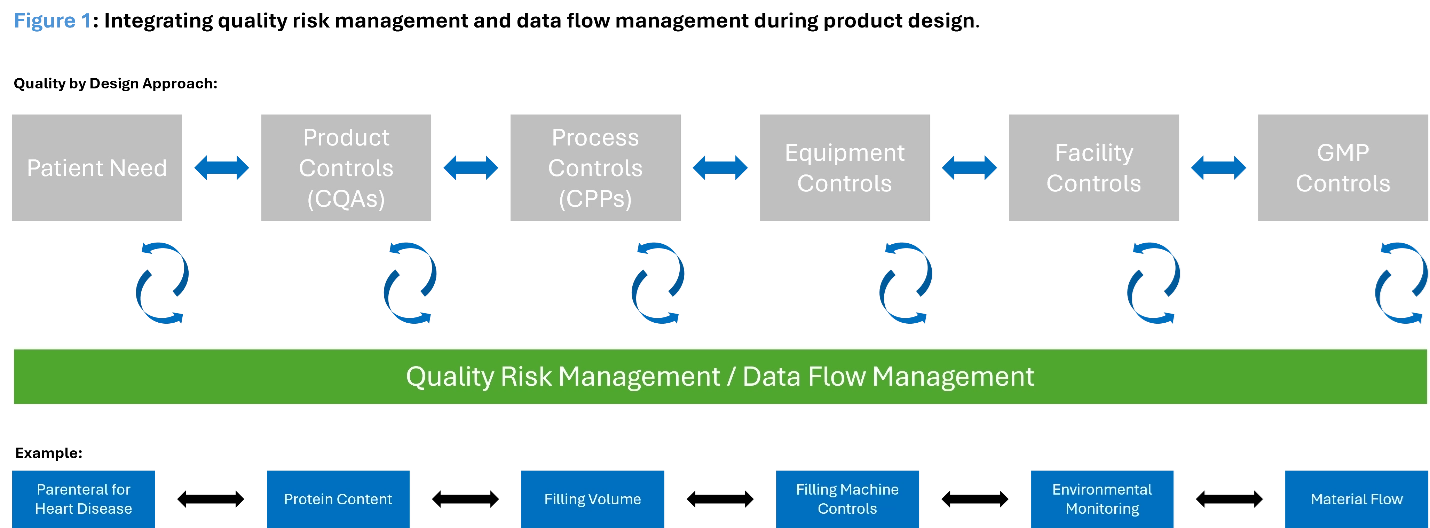
Benefits of Combining Validation 4.0 With Digital Technologies
When combined with digital validation technologies (DVTs) and emerging tools like ML and generative AI, Validation 4.0 offers significant advantages. These technologies enable a shift from traditional, document-centric validation processes to a more dynamic, data-centric, and real-time approach. This transformation allows for more efficient validation, where data can be continuously monitored and analyzed, driving faster and more informed decision-making.
Accelerating Validation Documentation With Digital Tools
Many life sciences companies have invested in the digitalization of their validation processes. According to a recent survey by the International Society of Pharmaceutical Engineering (ISPE), 74% of participants expect to implement a DVT by 2024.1 These DVTs enable faster documentation generation and integration with other critical systems, such as quality management systems (QMS), change control systems, laboratory information management systems (LIMS), manufacturing execution systems (MES), and data lakes containing CPP data. Using application programming interface (API) technology, which many of these systems support as they are SaaS-based, allows seamless integration between platforms.
This capability empowers organizations to transfer data between systems more efficiently and retrieve critical validation information in real time. For example, instead of waiting for a paper-based lab report on critical quality attributes (CQAs) for a process validation report, the system can automatically pull the approved results directly from an integrated LIMS in real time.
Enhancing Data Flow With AI and Machine Learning
The seamless and verifiable flow of data can be further optimized with the integration of generative AI and ML, which accelerates documentation generation and improves overall efficiency. Additionally, ML can actively monitor for changes that impact the control strategy, providing real-time notifications to users when adjustments are needed to maintain compliance and ensure a state of control.
Leveraging AI for Technical Document Creation
A key use case for AI in quality management is the efficient production of technical documents, such as validation protocols. This is achieved using a large language model (LLM) trained specifically to generate validation documentation in accordance with an organization’s standards and requirements. When paired with a retrieval-augmented generation (RAG) AI system, the LLM can ingest technical details from sources like user requirement specifications and equipment specifications to develop validation tests. Essentially, the RAG functions like a validation engineer, interpreting requirements, aligning them with equipment design, and creating test scenarios to verify if the equipment meets the required specifications.
The accuracy of the AI-generated output is verified by an operator who reviews and approves the test documents following existing paper-based workflows, which minimizes risk. Privacy concerns related to using an open AI model are mitigated by deploying the model on secure, private servers, ensuring controlled access and safeguarding data privacy.
Taking Validation 4.0 to the Next Level
In the future, combining DVTs with ML will unlock even greater automation opportunities. Imagine a scenario where a change to a user requirement affects the control of a CPP. In this case, AI technologies could automatically generate a test document to assess the effectiveness of that change. Once the document is approved, testing can proceed more efficiently by pulling relevant evidence from systems interfaced with the DVT, seamlessly integrating the results into the validation report.
This is a crucial step toward the ultimate goal of Validation 4.0: real-time verification.
More Uses for AI in Quality Management
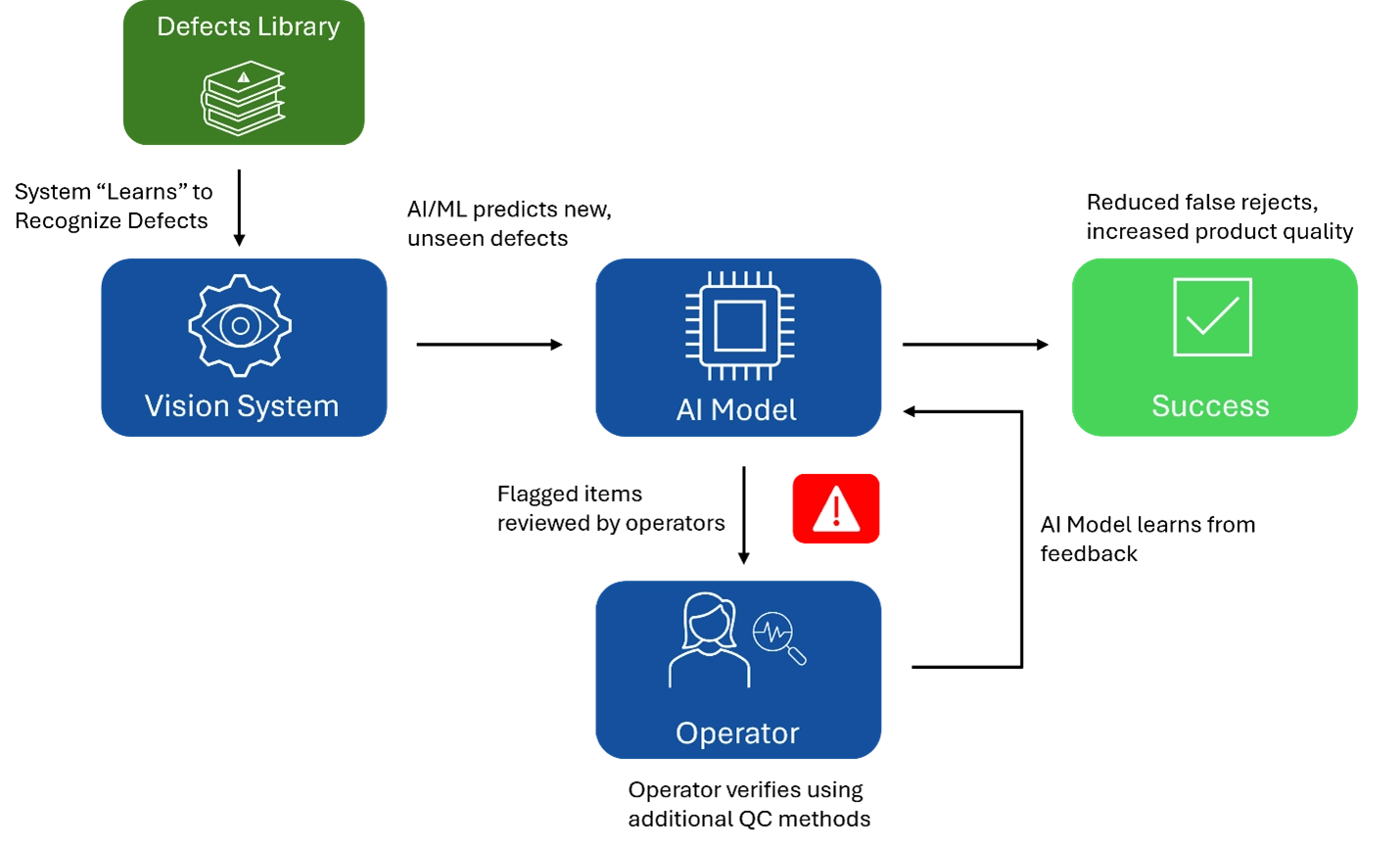
Other valuable use cases for AI in quality management can be found in quality control (QC) on medical device production lines, where vision systems inspect for defects. These systems are trained to recognize defects using a library of images, but it is difficult to simulate every possible defect. AI and ML can enhance these systems by predicting new, unseen defects and flagging them for operator review. The operator can then use parallel QC methods to verify whether it is truly a defect. This feedback loop allows the AI model to learn and improve, reducing false rejects and increasing overall product quality.
This approach not only strengthens medical device validation but also ensures the accuracy of AI/ML outputs, further improving the quality assurance process.
Discover How to Put Generative AI to Work
If you're interested in exploring how generative AI and validation software can address the accuracy and privacy needs of the life sciences industry, join us on October 16 at Masters Summit 2024 in Salt Lake City. We'll be presenting on how pharmaceutical manufacturing and quality professionals can leverage these tools to achieve Validation 4.0, with a focus on real-time verification and seamless integration within digital ecosystems. We look forward to seeing you there!
References:
- “2024 State of Validation Report,” Jonathan Kay, ISPE.org, Sept. 1, 2024.


