
4 Reasons to Adopt Digital
Production Records Now
Picture a manufacturing operation where you consistently achieve your key performance indicators (KPIs), routinely deliver products on schedule, and meet or exceed customer satisfaction metrics. The staff and company culture are aligned with your corporate mission, and your organization is recognized as an industry leader, putting your brand value on a steady upward trajectory.
How closely does your organization align with this description? The fact is, this type of operation already exists for many life sciences companies today. You can build this ecosystem and benefit from:
Holistic, real-time visibility.
Digital production records integrate with all other enterprise management systems.
Access to real-time data.
Market demand and resource management calculations can be forecast with precision.
Reduced operating costs.
Process automation eliminates errors, product rework, and scrap.
Complete digitization.
Employees are empowered by value-enhancing connections that have been established between enterprise systems, processes, and teams.
An edge in the market.
You can quickly gather and analyze data — enabling review by exception, which gets your products to market faster.
The Future of Manufacturing Is Here
This is an era of connectivity, advanced analytics, and automation. Companies embracing digital transformation:
- Understand the value of a manufacturing execution system (MES) and electronic production records on all product lines and in every site.
- No longer have to manage and prepare stacks of paper reports for audits.
- Increase operational efficiency and performance.
- Get products out to patients and customers faster.
What Does Digital Manufacturing in Life Sciences Look Like?
To better understand where companies are in terms of digital manufacturing operations, MasterControl surveyed 152 life sciences professionals in varying roles and focus areas. In short, 85% of your industry peers are already implementing advanced technologies.
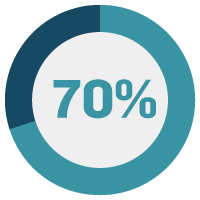
Digital transformation has become a strategic imperative.
If you’re not making the transition:
You risk falling behind your competitors.
Your operating costs will continue to increase.
You risk forfeiting your ability to get products to patients.
Your market position will decline.
Let’s take a deep dive into the advantages of digital production records over a paper-based manufacturing operation. We’ll also examine common obstacles to digital transformation and how companies overcome them.
1.
Drive Company Growth and Scalability
Efficient processes are a prerequisite for any company to continue achieving its growth and scalability goals. Capitalizing on a digital transformation requires a strategy that includes:
-
Advanced data analytics.
-
Real-time operational intelligence.
-
Smart manufacturing.
Companies achieve a high level of efficiency in their operation by implementing an MES. However, MasterControl research found that one of the leading barriers to digitization is the perceived cost of a system upgrade. This is a dated perspective in an increasingly digital, patient-centered life sciences industry.
By the Numbers
Digital transformation does bear a cost; however, too often companies fail to consider the actual cost of a manual operation. We found that year-over-year an inefficient manufacturing operation costs significantly more than implementing a digital solution. Around 80% of production issues are due to human error. The financial loss of deficiencies is measured in the costs of materials and other manufacturing resources, lost sales, and monetary penalties. In general, which can add up to tens of millions in spending with no returns.

Digitization takes these costly errors out of the equation. Using digital production records leads to:
- More efficiency.
- Fewer errors.
- Lower operating costs.
- Higher levels of productivity.
In addition, employees that would normally be spending time reviewing and reconciling paper reports, transferring documents to electronic format for audits, and locating missing data could spend their time on more business-critical tasks. The resulting cost savings is an investment in the company’s growth and scalability.
MasterControl’s Manufacturing Excellence is a modern, light MES that streamlines your manufacturing operation. Fully digitizing production records reduces manufacturing cycles, lead times, and review periods.
SPOTLIGHT
Regenerative medicine company BioBridge Global routinely worked with 400- to 500-page batch records with more than 2,000 entry points. Errors were common, resulting in deviations and compliance issues. The stakeholders realized that a digital solution could solve many of their problems. With MasterControl Manufacturing Excellence, the company’s batch records are error-free, so staff can prevent undetected issues from progressing through the manufacturing cycles.
Using electronic batch records (EBR) minimizes errors, allowing our scientists to focus on the work and not have to worry about entry errors because they’re able to do it all in an electronic system.
2.
Improve Organizational Collaboration
Digital manufacturing software embeds connectivity and intelligence into your manufacturing ecosystem. Gathering and analyzing data in real time enhances performance from raw materials to finished products.

In the survey, 41% reported that improving connectedness was the impetus for implementing an MES and using digital production records.
Business units within an organization, such as quality and manufacturing departments, can no longer have competing priorities. Stakeholders across the organization need to be unified to optimize operations and stay ahead of the competition. Still, some companies are hesitant to pursue a digital transformation due to the time involved in a system implementation.
By the Numbers
A traditional MES takes two years or longer to set up, configure, and incorporate into a company’s manufacturing operation. This type of MES is also costly and cumbersome — typically starting at $1 million — making it less economically feasible to deploy on all lines and in all sites.
MasterControl’s modern, light MES takes as few as 90 days to implement and digitize all your existing processes, making it feasible to use on all your lines and in all your sites. You improve right-first-time production, resulting in fewer products getting reworked or scrapped.
SPOTLIGHT
During the COVID-19-induced global lockdown, sterile compounding company QuVa Pharma was in the process of implementing MasterControl’s Manufacturing Excellence solution. Because companies still operated in a virtual working environment, every process from user requirements through go-live was completed remotely within three months using cloud technology.
With COVID-19, the benefits of Manufacturing Excellence are even more clear. We need to automate processes as well as share information, collaborate, and get approvals remotely. Having a system that allows us to do that is critical.
3.
Boost Productivity Metrics, Brand Value, and Revenue
Digitization eliminates the need to devote time and resources to production paperwork, change control, and problem resolutions. You dissolve departmental silos, putting you in a better position to:
-
Improve material tracking.
-
Shorten production cycles and reduce lead times.
-
Minimize quality issues and product recalls.
Still, some companies are concerned that a digital transformation will be too lengthy and disruptive to operations.
By the Numbers
A lot of companies still have an outmoded view of what digital manufacturing looks like. For economical reasons, a traditional MES is commonly used only on high- priority lines.
MasterControl’s MES has no-code configuration. You adapt it to your operation and processes, so digitized production records emulate what your operators are used to seeing and working with, resulting in minimal business disruption.
SPOTLIGHT
High-end vascular catheter remanufacturer Northeast Scientific needed a way to scale its operation to meet the growing demand for its products. Digitization was the next logical step.
Before, we did one 510(k) a year. Right now, we’re doing four or five 510(k)s simultaneously. Now we’re competing at a level we should be.
4.
Elevate Return on Investment (ROI) Metrics
Speed, quality, and safety are high priorities in an industry where there is little to no margin for error. A manual, paper-based process or traditional MES solution won’t have the agility or computing power needed to oversee critical aspects of manufacturing, such as:
-
Monitoring and synchronizing manufacturing activities across globally distributed departments.
-
Collecting data, creating reports, and delivering manufacturing instructions to the shop floor.
-
Reducing scrap and rework by eliminating manual entry errors.
However, executive leadership might be reluctant to invest in the type of infrastructure that optimizes manufacturing and keeps the company aligned with current manufacturing trends.
By the Numbers
With MasterControl’s modern MES, companies recognize ROI within four to eight months, an average savings of $300,000 in the first year, and a savings of over $2,000,000 in the first five years. With this ROI, it’s easier to make the case to leadership about the advantages of digitizing manufacturing operations.
SPOTLIGHT
Pharmaceutical compounding company Fagron struggled with documentation updates. The quality management teams spent nine hours every week printing and binding up to 300 pages of training materials. The documents then needed to be autoclaved to be used in the laboratory and clean rooms. With MasterControl, all the training materials are electronic, which saves hundreds of hours and thousands of dollars a year.
I would say MasterControl has made our life so much easier. Now we can put our focus more on bringing our products to people.
Why You Need a Modern Manufacturing Software Solution
It has become essential to seek out ways to scale, improve processes, and reduce lead times. MasterControl’s light MES is a paperless, scalable system for efficiently managing manufacturing operations.
Digital production records.
Includes error-reducing features that prevent mismarks or missing information on batch records.
Equipment maintenance.
Manages the equipment maintenance schedule and automatically generates preventive maintenance tasks before they’re due.
Review by exception.
Eliminates line-by-line reviews. Simply review exceptions, easily identify issues, and fix data-entry errors in real time.
Where does digitization rank in your company’s business strategy? Determine your current status in the digital maturity model by taking our digital maturity assessment.
Start Your Assessment