
The Ultimate Guide to Pharmaceutical Quality Management
1.
Shifting Focus:
Adopting a Data-Centric Quality Mindset
Visualizing a new data-centered quality paradigm.
When the first Nokia and Motorola cell phones that were affordable and small enough to fit in your pocket hit the market, they not only changed how we viewed phone technology but how we communicated.
Mobile phones went from a novelty that only the wealthy could afford to an indispensable device that virtually everyone has and, in many ways, can’t live without. But unfortunately for both Nokia and Motorola, the transformative effects of cellular devices were not at their apex. In fact, the cycle of innovation was only getting started.
The introduction of the iPhone took things to the next level by fundamentally shifting the value of the cell phone from voice to data. By opening new features like text and video messaging, internet access, and other seemingly limitless avenues for the usage and access of data, the iPhone dramatically increased the utility and value of a mobile device and forever changed the way we live, work, and play.
Innovative technologies have had a similar impact in pharmaceutical and biotech industries. Much in the same way that cell phone technology revolutionized modern life, digital quality management system (QMS) solutions have transformed paper-based quality and compliance processes for the largest pharma enterprises, startups, and every type of organization in between. They provide modern digital tools for managing and automating quality and compliance documentation to create efficiency, increase productivity, and mitigate risk.
Nowadays, similar to the way the iPhone shifted the value from voice to data applications, an innovation revolution continues to unfold in the pharma world. A fundamental shift is taking place in the way companies approach quality and compliance. The focus of quality management is moving beyond digitizing documentation and embracing an approach that capitalizes on data-driven insights.
An increasing number of discerning pharma companies are steering away from quality management methodologies that focus on “data objects” like documents and instead embracing a model that enables more granular access to all the information contained within them. Advanced technologies are entrenching themselves as the cornerstone of this new approach. Pharma companies that have already implemented modern digital tools are now more capable of connecting and analyzing data across product life cycles and using it to predict issues and prescribe preventive actions.
Much like the iPhone didn’t eliminate voice applications, this shift is not about replacing documents and processes. It’s an additive evolution that is progressing toward the seamless connectivity of all quality and compliance data across the entire life cycle of a pharmaceutical product. As this evolution continues to gain momentum, quality data is becoming even more valuable to pharma businesses as they struggle to shorten development timelines and get products to market faster — all without jeopardizing compliance.
That’s why it’s necessary to take things a step further by embracing reliable modern tools that can enable pharma companies to contextualize and make better use of their data. Applying advanced analytics and artificial intelligence (AI) tools to data usage requires a proper foundation, however. Accuracy in analytics and AI is dependent on the veracity and completeness of the data in use. Unfortunately, most pharma companies have a bad habit of storing that data on paper, in employees’ computers, or other similarly siloed locations. Even companies that have digitized quality processes with an electronic QMS can have trouble merging and deriving meaning from all their data.
Bringing together information from every department in the organization and having the tools to properly analyze it are critical to survival in the hyper-competitive pharma industry. The digital documentation layer of quality and compliance processes is still essential and will never go away, but advanced technologies are helping pharma organizations unlock the data side of the equation.
The promise and peril of unstructured data.
As they say, the first step is admitting you have a problem. The primary driver behind the medical device industry's urgency to adopt more data-centric approaches to quality and compliance is best understood through the lens of unstructured data, which accounts for more than 80% of data in the life science development, production, and commercialization life cycle. 1 Think about locked PDFs, scanned documents, uploaded images, and so forth — all elements that can be “managed” within today’s QMS software solutions, but all elements that contain massive amounts of granular data and insights that are currently difficult to extract and hard to correlate and analyze in real time.

Nothing can be done with unstructured data until it is converted into a useable format. Then, pharma companies can focus on data integrity and improving data quality. This is one of three critical points to understand about the hazards of unstructured data and its impact in the evolving regulatory landscape.
- Poor data quality is a multimillion-dollar waste.
There’s quality data, and then there’s data quality. Quality data is simply data collected through quality control and quality assurance efforts. Data quality refers to how good the data is and largely ties back to following good data integrity practices. Ignoring those practices and settling for poor data quality costs organizations an average of $12.9 million annually, according to Gartner. 2 Pharma companies have data that can give them advantages over their competitors — if they can access it. The first step in access is removing any practice that leads to unstructured data.
- Data will play an increasingly important role in regulators’ policy enforcement.
As regulatory bodies strive to extend their global alignment and coordination, they are adopting new data-driven approaches and increasing data sharing and harmonization. With each new regulation that goes into effect or standard that is updated, regulators are placing increased emphasis on risk, shifting from a one-size-fits-all regulation mindset to a data-driven, segmented approach. This is evident in how the U.S. Food and Drug Administration (FDA) chose to handle inspections during the pandemic. The agency used risk management methods to determine when to request a remote interactive evaluation. Being able to access data quickly has always been important during inspections, but for the FDA’s remote records review having it in an electronic format was essential.
- Unstructured data is a massive blind spot in today’s quality and compliance models.
An approach to compliance that focuses on the object that contains the compliance information that regulators seek (i.e., a document) is insufficient in the current regulatory environment. In the eyes of regulatory authorities, quality evidence that cannot be substantiated upon request may as well be nonexistent. Conversely, achieving an audit-ready state that is sufficiently supported by the appropriate digital technology is inherently more efficient and would drastically lower overall compliance costs. Combining audit readiness with effective compliance risk assessments and management would further improve the effectiveness of compliance functions. But adopting this type of approach to quality and compliance requires that a pharmaceutical company first overcome the obstacle of unstructured data.
Overcoming data overload.
More data, more insights, more opportunity — got it. Not so fast. Like their peers in R&D and marketing before them, pharma quality professionals can quickly find themselves inundated with too much data. Besides being flooded with machine performance, product performance, process performance, and observational data, they also have access to “internet of things” (IoT) data, social media data, structured customer feedback, and human sentiment data. With this deluge of available data, the expectation is that quality should be able to do more with it. But turning this data something actionable is challenging.

$67.82B
Projected value of big data analytics in the health care market by 2025 3As traditional health care companies struggle to tap into their unstructured data, large tech companies are rising to the challenge. The Google Cloud Healthcare Data Engine and Amazon HealthLake are frontrunners in this area. 4 Both efforts have similar goals in analyzing unstructured data and overcoming the interoperability issues that are common with electronic health records.
Pharma companies that cling to their antiquated document-oriented approaches to quality are just going to fall further behind big tech competitors like Amazon, Apple, Google, and Microsoft who are positioning themselves to be major competitors in R&D, drug manufacturing, clinical development, and other pharma spaces. A pharma company’s failure to invest in software solutions that improve data collection, management, and analysis while simultaneously improving the analytics comfort and capabilities of quality professionals will at best only result in inefficiencies, production delays, or product defects. At worst, it’ll lead to the harsh consequences of noncompliance or product recalls.
Today’s QMS solutions are a giant step forward from antiquated, paper-based quality management processes, but they have the capability to be so much better. Getting there requires moving beyond merely digitizing document-centric processes and focusing on a holistic approach that allows quality and compliance professionals to access, analyze, and apply insights from structured and unstructured data within the same system across the product life cycle.
Understanding quality’s document dependency.
No self-respecting tech company would ever record and store important information on paper. Even if big tech wasn’t a potential competitor, nixing the bankers boxes and filing cabinets is still a good idea. But the document-centered approach to quality management has been the default mindset in the pharmaceutical industry for as long as it’s been regulated. The mentality has been predominantly based on the rationale that quality activities are typically rooted in historical information that is maintained in reviewable documents.
And while there is a rich history of statistical data analysis in quality, the traditional models have typically employed point-in-time analysis that provided parameter guidance based on past quality events. The conventional approach is not effectively supporting pharma companies as they find themselves generating ever-growing collections of disconnected documents and data. And those document collections and disconnected data sources are multiplying and fragmenting exponentially. What’s more, regulatory agencies are recognizing that the one-size-fits-all approach to regulation does not adequately weigh the wide range of risks posed to different pharma companies, which has triggered the subsequent shift to a data-driven, segmented approach to regulation.
Noteworthy perils of disconnected documents include:
- Inefficiency
It’s difficult to connect documents to multiple processes, and disconnected documents and processes increase the likelihood that quality issues will slip through the cracks. Compound those inefficiencies with the fact that staff time equals money in any business setting. The time employees must spend controlling versions or determining the accuracy of disconnected documents equates to resources that a pharma company can’t allocate to other critical areas.
- Innovation Blind Spots
When the right hand doesn’t know what the left hand is doing in a pharma company, it can be a fatal flaw. If you can’t get the right document in the correct person’s hands when decisions need to be made, your quality data is useless — especially to regulators.
- Operational Disconnects
To have any hope of managing product life cycles effectively, your workflows and document approval routes need to be as straightforward as possible. But in document-focused environments, the management of life cycle statuses and changes becomes incrementally more complicated when modifications inevitably occur or whenever new products are introduced.
Charting a clear path to digital and data maturity.
Every pharmaceutical company’s quality system can be identified at a specific point on the QMS maturity curve. And it’s likely that every pharma company is at least one step behind where they’d like to be. Where is your company? Where would you like to be? And, more importantly, what do you need to do to get there? Achieving true quality data intelligence requires that you evolve your document-oriented quality mindset and systems to become more focused on the availability, connectivity, and analysis of your data.

Taking pragmatic steps toward digital leadership.
There’s only so much an individual can do without the approval and support of the C-suite. Digital transformation is best done holistically, not piecemeal, and that level of vision and planning requires someone further up the organization’s hierarchy. Digital transformation, connected data, and AI are just steps on the same continuum to make better decisions based on data with less error.

McKinsey & Company performs an annual survey about AI, specifically looking at the difference between what they refer to as AI high performers and other organizations.
“The most successful companies we see have a CEO who lays the groundwork for support up front. Such leaders … hire AI-experienced senior talent to fill the leadership positions required to help drive the change, if the talent doesn’t already exist in the organization. They also reduce hierarchy, make AI education a priority, and consistently communicate at every level the strategic nature of these changes.” 6
If your company isn’t yet planning any AI initiatives, now is the time to start. AI, especially machine learning (ML), is playing an increasingly vital role in quality process optimization. There isn’t a single pharma company that couldn’t benefit from the enhanced predictive/prescriptive analytics that AI can provide. But undertaking an AI initiative isn’t as scary as it sounds. There’s no need to run out and hire AI experts when advanced tools with built-in AI are already on the market.
Connecting the 4 Ps: People, Processes, Policies, and Platforms.
At MasterControl, we are actively focused on the future and helping our customers get the most out of their quality and compliance data and processes — not just at a technology level, but at the operational level. Digitization continues to be the overarching trend in the pharma industry, and quality leaders are learning that they must find new ways of reimagining and redesigning their processes in order to become more focused on managing data digitally.

Overhauling processes is hard enough without worrying about the technology involved. That’s why we’re working to integrate completely connected quality management and predictive and prescriptive insights into process improvements. With an intuitive user interface, MasterControl products help employees embrace digital transformation and advanced technology such as AI by making it easier to use, which also helps their organization achieve ROI faster.
As one expert from Deloitte points out, “With AI capabilities increasingly embedded in enterprise software, and an abundance of cloud-based offerings and tools that accelerate AI development, a company no longer needs as many heavy-duty specialists to get started.” 8
We know how valuable analytics and AI are to pharma organizations, which is why we’re building those capabilities into our products. Pharma companies can immediately reap the benefits, even as they work to transform their whole organization.
The future of connected quality.
Speaking of analytics and AI, that’s really where the quality field in general is headed. That doesn’t mean quality professionals have to go back to school to get new degrees, but it does mean becoming familiar with the tools available and the benefits they offer. By automating the more tedious tasks associated with quality management, quality professionals can focus on improving the pharmaceutical products themselves and getting them to market faster.
As additional benefits of connecting data on a granular level continue to arise, it becomes easier to visualize scenarios where the power of quality data can be unlocked and harnessed.
Predict overdue training and provide options for how to avoid it.
Recommend updates to training based on user error during production.
Track and trend themes across customer complaints using natural language processing (NLP) applications.
Employing specialized algorithms that can determine when investigation is warranted, carry out much of the legwork of an investigation, and in some cases, close the investigation with little or no human involvement.
Use industry data to create benchmarks and measure where a medical device company is underperforming.
Predict the time delay of a deviation on a product’s release.
While new technology creates new challenges, it also opens the door to new opportunities. The following section and subsequent chapters describe how you can make data-centric quality a reality in your organization by implementing tools and methodologies that fully connect the entire quality life cycle, extend the quality ecosystem, and unlock the hidden intelligence and predictive capabilities that are waiting to emerge from your data.
Modernizing quality’s approach to data.
As previously mentioned, quality has always been driven by data in the pharmaceutical industry and, as regulations and standards continue to be updated to match the pace of the modern digital world, it’s never been more important for quality teams to make data-driven decisions.
While quality has always been the leader in statistical modeling and earlier forms of data-driven decisionmaking, those developments largely occurred in an era when accessing and analyzing data in real time with prescriptive and predictive models wasn’t an option. Today, most of the market is lagging in its use of up-to-the-minute metrics that facilitate better and faster decision-making.
Without access to pertinent quality data, pharma companies will fall behind their better equipped competitors. A new approach requires a new set of tools, however. That’s why MasterControl solutions are designed to support your transition from document-centric to data-centric quality and compliance management.
MasterControl’s Connected Quality Advantage.

To provide even more connection between quality data and other areas of the organization, we have developed MasterControl Insights. Insights is our analytics tool that incorporates predictive/prescriptive analytics and AI to guide your decision-making. Insights gives users complete control over their data to decide what’s important to their specific role and how to visually display it in the most meaningful way. Data’s potential is too important to not embrace new technology. Even regulators agree with that.
The FDA’s recently established Office of Digital Transformation (ODT) is evidence of this. “The agency began these efforts because … innovation is at the heart of what we do. By prioritizing data and information stewardship throughout all of our operations, the American public is better assured of the safety of the nation’s food, drugs, medical devices, and other products that the FDA regulates,” said former Acting FDA Commissioner Janet Woodcock. “This reorganization strengthens our commitment to protecting and promoting public health by improving our regulatory processes with a solid data foundation.” 9
2.
The Platform Effect:
Fully Connecting the Quality Life Cycle
Combating complexity with connectivity.
Pharma companies are facing intensifying pressure to accelerate new product introduction pipelines while being simultaneously confronted with decreasing margins and products that are becoming more elaborate and personalized. This is resulting in increasing scrutiny on the industry’s siloed approach to systems and processes.
As the industry strives to adapt to the market’s velocity, three key trends have emerged as impediments to many companies’ attempts to connect the quality life cycle:
- Diminishing timelines
It’s no secret that the pharma companies that have undergone digital transformations are moving faster than those still using paper. But with more and more tech-savvy companies joining the fray, getting to market faster has never been more critical. McKinsey & Company experts propose the traditional approach to drug development is due for a holistic transformation. “While there is no silver bullet, drug developers can make a concerted effort to apply and integrate multiple innovations that can transform development,” according to McKinsey’s industry researchers. “In our estimation, it should be possible to bring medicines to the market 500 days faster, which would create a competitive advantage within increasingly crowded asset classes and bring much-needed therapies to patients sooner.” 10
It should be possible to bring medicines to the market 500 days faster, which would create a competitive advantage within increasingly crowded asset classes and bring much-needed therapies to patients sooner. To transform drug development, this acceleration can be combined with improved quality and compliance, enhanced patient and health care-professional experience, better insights and decision making, and a reduction in development costs of up to 25%.
- Product complexity and diversity
In the age of gene therapies and precision medicine, pharmaceutical products have never been more complex. And with these types of specialty pharmaceutical products expected to account for nearly two-thirds of new product launches in 2023, complexity will only become more rampant. 11 Accompanying this upsurge is the fact that the products developed by pharma and biotech companies are also growing more diverse and have shorter product life cycles than ever before. Concurrently, the industry is experiencing an increasing emphasis on outcomes and new delivery models. As pharma products become more diverse and intricate, the reliability and accessibility of the quality data that supports them must increase in equal measure.
- Personalization
Personalized medicine continues to be a significant disruptor in the pharmaceutical industry. The widening field of patient-specific therapies is resulting in an explosion of quality data. To handle larger numbers of patients served each year and avoid becoming bottlenecks, quality control and quality assurance functions will need to be more automated and demonstrate continuous real-time capabilities. Automation is only one part of the equation, though. Any technology that is implemented to streamline quality data management must also be capable of making that data accessible and relevant across the enterprise.
The next level of quality culture.
The demand for acceleration and the enhanced focus on data is bringing purpose-built software to the fore in the pharma industry. It’s no longer enough to simply have a document control system or a quality event management (QEM) system. Pharma companies need greater real-time visibility into data and more control over quality processes, especially as complexity increases and the pace of innovation accelerates. This is only found in systems that connect all areas of quality — training, document control, audit, QEM, etc. The best systems reach even beyond quality to glean information from and share data with other departments within the organization.
Rather than piecing together disparate applications to coordinate multiple data streams and processes, integrated software offers the reassurance of native connectivity and unifies all applications and processes within a common architecture and database. Plus, a pharma company can achieve greater governance over its quality management processes by running multiple applications within a natively integrated operating system. This, in turn, allows the organization to take a product life cycle approach to quality and compliance. Such an approach pulls data together not only from the different areas of quality but from the entire organization.
Advanced analytics let pharma companies operate based on the accrued data and measure their progress in fast, reliable ways. When quality professionals spend less time doing data dumps into Microsoft Excel, they can focus on the stories the data is telling them and act on the suggestions of the prescriptive algorithms. The time saved through digitizing and relying on advanced automated tools lets pharma companies move faster, improve agility, and outpace their competitors.
Fuel Growth With a Connected Platform

Enabling vision and velocity.
Greater speed comes down to vision — leaders who can see what’s happening in real time can make decisive actions quickly. This was never more important than during the pandemic. COVID-19 forced companies to change how they work, and pharmaceutical and other life sciences companies were no exception. “Companies shattered previous vaccine development records due to their ability to capture, store, process, and analyze machine data,” said Deloitte Principal Aditya Kudumala. 12 By enabling companies to achieve real-time quality intelligence, modernized digital systems proved to be the ideal means of establishing quality as a speed-to-market accelerator.
If you want to understand why [data] is incredibly relevant to life sciences companies, look no further than COVID-19 vaccine development. Companies shattered previous vaccine development records due to their ability to capture, store, process, and analyze machine data.
As pharma companies continue to recover from the pandemic's effects and focus on growth, digitization provides adaptability and expansion when the need to evolve or pivot becomes necessary. While not as urgent as COVID-19, business processes change and new processes need to be added. A robust digitized system equips an organization for the future by providing the consistency of a baseline technical framework that can be expanded upon and leveraged to catalyze innovation as business flourishes.
A recent report from Cognizant looked at the effects of rapid digitization on the life sciences industry. “In the post-COVID-19 era, ‘digital’ will mean more than just applying technology to business processes; it will mean reinventing the business by connecting data, knowledge, people, and insights, turning traditional life sciences into a proactive industry.” Cognizant’s research indicates that digital transformation will continue as life sciences companies must adapt to keep up with competitors and provide better products and services.
The same report included research indicating how far along (or behind) life sciences companies are in their digital transformation and where they’re hoping to be in the future. Some interesting points include:

If a pharma company isn’t at least planning on implementing these technologies, now is the time to start. “Life sciences, we now know beyond a doubt, is far more agile than anyone could have imagined,” said Cognizant Chief Digital Officer of Life Sciences Brian Williams. 13
Connect with confidence.
MasterControl customers demonstrated their agility demonstrated their agility during the pandemic. Early in the outbreak, leading pharmaceutical compounding and personalized medicine company Fagron had to ramp up its processing of the relatively common steroid dexamethasone to keep pace with rising demand.
The global company is frequently called upon by compounding pharmacies during drug shortages, and the pandemic significantly increased the pressure on the provider to boost available quantities of the steroid. With the robust yet flexible capabilities provided by MasterControl’s cloud-based QMS, Fagron was able to meet the elevated demand, according to the company’s North America quality specialist, Matthew Seitz-Paquette.
“Dexamethasone is something that we’re able to source and supply to compounders around the world, but it’s something that a large organization in traditional pharmaceutical manufacturing may struggle with,” said Seitz-Paquette. “Being in the cloud, we’re able to organize sourcing and documents and coordinate everything from our qualifying and auditing teams in China, Europe, South America, and North America very rapidly.” 14
A proven track record of excellence, a commitment to customer success, and an emphasis on innovation have made MasterControl the platform for quality. With more than 1,100 customers around the world, MasterControl has established itself as the leading quality platform provider in the field. MasterControl has also earned the trust of regulatory authorities, many of whom — including the FDA — use MasterControl solutions to meet their own rigorous internal standards for quality management. Companies and regulators alike rely on MasterControl to connect essential data and extend quality beyond just the quality department.
With MasterControl ensuring that all your quality and compliance data is maintained in one location, your entire organization can move faster and be more responsive. Our solutions give you the tools you need to increase profitability and maximize business intelligence.
3.
New Ecosystem Dynamics:
Enabling Pervasive Quality
Maintaining quality in the era of outsourcing.
As a result of the rising timeline pressures, augmented complexity, and distributed supply chain factors discussed in the previous chapter, pharma companies are increasingly adopting asset-light models that are heavily reliant on a broad ecosystem of design, research, and manufacturing organizations. To achieve their goals, an ever-increasing number of pharma companies are widening their networks of contract manufacturing organizations (CMOs) and contract development manufacturing organizations (CDMOs).
Establishing solid relationships with reliable contract partners can bring dramatic improvements in two key areas that are traditionally problematic for pharma organizations:
Flexibility:
74% of quality process end users report that launch delays of products and services are frequently caused by inflexible quality processes. 15Control and transparency:
60% of organizations struggle with real-time visibility into CMO production and 40% lack control over product quality. 16

$276.8B
The global pharmaceutical contract manufacturing and research services market size is projected to reach by 2028. 17Even so, maintaining dynamic, asset-light operations and outsourcing increasingly more functions means that quality departments must find new ways to enhance flexibility and extend collaborative efforts across a growing number of external partnerships — without increasing risk.
For pharma companies to successfully outsource more functions, there’s no escaping the fact that quality cannot effectively operate in isolation. Quality must be consistent and pervasive throughout the supply chain in order for it to be managed comprehensively and for compliance to be sustainable. To maintain comprehensive control and complete oversight over quality, you must have continual visibility into all quality activities as they’re occurring throughout the entire ecosystem.
This requires not only a platform that enables you to digitize, automate, and connect quality processes internally, but one that leverages modern integration management capabilities. That’s why MasterControl designs software solutions with integrations in mind. Our software solutions offer simple and reliable connectivity of critical data between enterprise systems — providing the visibility and control our customers need to ensure quality and maintain compliance.
MasterControl’s integrations enable seamless data exchange between systems like:
MasterControl’s integrations enable seamless data exchange between systems like:
Laboratory Information Management Systems (LIMS)
Manufacturing Execution Systems (MES)
Enterprise Resource Planning (ERP)
Supply Chain Management (SCM)

Key questions to ask about QMS extensibility.
As your quality ecosystem matures, you need a QMS that promotes flexibility and responsiveness. To ensure your quality management competencies can keep pace with the dynamics of your ecosystem, consider the following questions when evaluating any solution that makes claims about its data and process management improvement capabilities.
Is it configurable?
Does it have the necessary modularity and integration features your business requires?
Does it incorporate risk-based analysis?
How quickly can upgrades, patches, new features, and enhancements be implemented?
How burdensome is validation?
Can the intelligence the system provides be personalized according to users’ roles?
Is it conducive to adapting to the requirements of technological and regulatory innovations?
Is it scalable enough to facilitate the future expansion of your business?
Market pressures are requiring pharma companies to have satisfactory responses to these questions.
As the regulatory environment grows, our customers are demanding more of us,” said Tony Harnack, president of nutritional supplement provider Wellington Foods. “They’re demanding more of their finished product and everybody’s liability and risk has increased. Everybody in the supply chain recognizes that. And everybody in the supply chain who wants to be doing this 20 years from now and who wants to continue to grow is investing in their quality management system."
Feedback from the executives and quality managers recently surveyed by BCG tells us that as qualityfocused organizations increase their QMS investments they are finding that successful implementation entails corresponding improvements to the management and quality of data, as well as enhancements to analytics technologies and methodologies. And the quality leaders who are committed to making these improvements are expecting — and getting — big returns in each area of the value chain.
The pharma organizations on the leading edge who are past the planning stages and have already begun implementing digital technologies are already realizing the important gains that can be made in the areas of quality governance, training, and performance management.
Extending quality’s reach while strengthening control.
A robust quality management system like MasterControl allows pharma companies to expand quality’s scope and impact beyond the quality department to improve efficiency and effectiveness in other areas of the organization.
Quality is a department, but it should also characterize everything a pharmaceutical company does. Hence the desire to expand quality’s reach. When multiple departments operate on the same system as the quality function, they can easily exchange information which extends quality far beyond the final check before a batch is shipped out.
MasterControl offers an array of unique tools that enable you to optimize and visualize your extended quality ecosystem:
MasterControl’s robust Supplier Excellence and Bill of Materials (BOM) solutions facilitate the expansion of and control over quality ecosystems for companies that are increasingly dependent on widening networks of CMOs and CDMOs. These comprehensive MasterControl tools allow companies to manage the supply chain from beginning to end and provide a centralized global repository for managing all contractor and supplier data, documentation, and workflow processes. This ensures pharma organizations and their authorized external associates have instant access to essential information from any location, at any time.
MasterControl Insights is our analytics platform that lets users create customized visualizations and tabular reports. It incorporates data from all MasterControl products, including data about documents, training, quality events, manufacturing, and more. Insights also has AI capabilities that use historical data to help companies operate more effectively. Ultimately, it lets users make data-driven decisions faster while tracking their key performance indicators (KPIs) so they can measure progress in real time.
MasterControl Manufacturing Excellence provides electronic batch records that simplify the input, management, and tracking of production data. Many pharma manufacturers that have implemented Manufacturing Excellence are seeing a 90%-100% decrease in data entry errors. Similarly, review times are reduced by up to 80%, letting them get product out the door that much faster. This saves time for everyone involved and provides manufacturing records that are more accurate, and their electronic nature makes them easy to locate during an inspection or audit.
MasterControl has the capacity to provide reliable enterprise system integrations with less effort, risk, money, and time required on your part. MasterControl integration capabilities ensure your quality data will have:
Consistency across systems.
Greater value.
Continual availability.
Sustained integrity.
Increased capacity to support and drive decision-making.
Extended reach across the enterprise.
SPOTLIGHT
Connecting quality data with the MasterControl platform has enabled Fagron, a global leader in personalized medicine, to replace binders full of training documents with tablets. Fagron’s quantifiable improvements since implementing MasterControl:

MasterControl has made our life so much easier. Now we can put our focus on making our products and bringing them to people.
4.
The Future of Quality Is Predictive:
Keys to Unlocking Your Data’s Intelligence
Going beyond proactive.
Shifting from a reactive to a proactive approach to quality and compliance has been the pharma industry’s de facto mission statement for years. Yet, in such a highly competitive industry, the ability to leverage data for predictive — and not merely proactive — purposes is becoming a must-have competency of the quality function.
Pharma companies that adopt a data-driven, platform-enabled quality model are dramatically improving their ability to yield real-time quality intelligence and predictive insights. With the capability of connecting data within a common platform, every function within a pharma organization — from the C-suite to the shop floor and every point in between — can have an appreciable impact on transforming product quality data into real-time intelligence. And market trends indicate that your competitors are fully aware of this. Investments in quality data and analytics enhancements are on the rise, with 40% of quality organizations reporting that they’re budgeting for data, analytics, and/or intelligence improvements within the next 12 months.
Making analytics and product quality data management improvements can yield big returns.
McKinsey & Company has developed an approach they’ve dubbed “smart quality,” which is a combination of improved processes, automation and digitization, and advanced analytics. It’s provided measurable benefits in many areas, including:
Accelerated review processes:
After leveraging tools that offered “smart” visualizations of cross-functional, connected metrics, one pharma company drastically improved the efficiency and effectiveness of quality management reviews at all levels and reduced late regulatory reporting to zero.
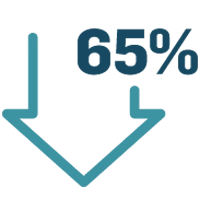
Reducing recurring deviations and nonconformances:
By applying innovative technologies to identify, explain, and eliminate root cause of issues, several pharma companies have reduced the overall volume of issues by 65%.
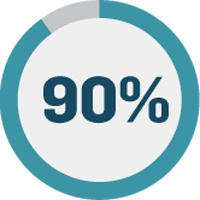
Shortened investigation times:
Along the same lines, investigation cycles for those deviations and nonconformances have been reduced by 90%. 19
Mixing proven platforms with emerging technologies.
At a foundational level, quality and compliance professionals are looking to modernize their core QMS systems. Gartner recommends evaluating providers against a mix of present state and future state considerations:
Define QMS software requirements by thoroughly assessing the current state of the quality organization and coupling that assessment with anticipated business needs in the future.
Consider compatibility and integration through application programming interfaces (APIs) for ERP, PLM, MES, LIMS, LMS, and other commonly used enterprise systems.
Evaluate providers’ and their partners’ industry depth, geographic reach, and product roadmap by asking detailed questions about each.
Consider the vendor’s product roadmap, strategic direction, and how it is differentiated from its competitors.
Gartner also strongly advises that companies thoroughly analyze their quality organization and processes before beginning the request for proposal (RFP) or request for information (RFI) process. Those in the market for a QMS are typically focused on “employee training, remote supplier audits, and the need for continuity with respect to handling [corrective action/preventive action] CAPA and nonconformance in a remote environment.” 20
Gartner clients report, and QMS solutions providers have observed, that quality management has moved outside the quality function and has more enterprise-level visibility. As a result, manufacturers have a heightened desire to avoid quality issues earlier in both the product design and manufacturing processes; they seek solutions that are increasingly focused on predictive qualities and proactivity, as opposed to containment and reactivity.
The intensifying need for remote work has highlighted the industry’s dependency on the cloud and cutting-edge technology. Some of the specific capabilities that tech-savvy pharma organizations are coming to expect from advanced QMS solutions include:
Improved access to essential, timely data:
During the pandemic, access to data from anywhere at any time was vital. Companies without remote capabilities floundered when it came to quality processes. “In the current environment, Gartner clients report significant challenges collaborating with colleagues in the quality organization, accessing data remotely, processing changes, and handling ongoing uncertainty.” Software that provides that remote access streamlines decision-making by giving the people who need up-to-date quality and compliance data quick access to it.
High-value uses for emerging technologies that advance quality goals:
Finding a way to convert product quality data into real-time intelligence and predictive insights was once just a fantasy. Thanks to the relentless advancement of technology, however, the predictive power of data has become the new reality — and it is gaining more relevance and generating more value every day. Advanced analytics are more an expectation than a perk, and AI will soon fall into that category as well. According to a survey from PwC, 88% of respondents in the life sciences said the pandemic accelerated AI adoption. 22Ease of use:
Increased competition in the software industry means vendors are tripping over themselves trying to improve their user experience. This is great news for pharma companies that are looking for QMS or other enterprise software. If a pharma company finds a particular software solution difficult to implement, use, validate, or update, there are plenty of other options that are likely a better match.
Fueling growth and innovation with connected data.
All pharma companies should be making data-driven decisions. That reality is within reach with modernized software solutions that offer advanced analytics and AI in their platforms. AI-enabled solutions are becoming so prevalent that a recent Deloitte survey found that 75% of AI adopters believe all enterprise applications will have built-in AI by 2023. The real competitive advantage AI provides, according to Deloitte Research Manager Susanne Hupfer, “may depend on organizations applying AI more creatively and responsibly.” 23
MasterControl is on track to incorporate AI into our software. We already ensure your processes and data are connected. MasterControl's integration options extend your quality management competencies to other business-critical applications while still safeguarding data synchronization between systems. With MasterControl’s extensive integration capabilities, you always have the assurance that your data has a single source of truth.
That data will be best used with our advanced research projects that are aimed at helping organizations effectively and confidently capitalize on emerging technologies like ML and AI; natural language processing (NLP); and intelligent process automation (IPA). Recent advanced research projects have focused on using advanced NLP, ML/AI, and process automation technologies to:
Automate text extraction from unstructured documents, identify and contextualize relevant data and auto-populate information into MasterControl to increase efficiency and accuracy.
Create Netflix-style contextual search and recommendation engines to improve search speed and pull relevant information and documents forward based on users’ past searches and search language history to refine the recommendations it provides over time.
Streamline workflow creation and execution and allow users to effortlessly connect data sources, process steps, and systems.
To keep pace with the pharma industry’s changing quality landscape, quality leaders can rely on proven MasterControl solutions that are engineered to help quality teams be more dynamic and work with MasterControl innovation teams to define the future of data-driven quality
The importance of a digitally empowered quality workforce.
The COVID-19 pandemic changed a lot of things in the general workforce. One of those was a shift in power from the employers to employees.
Another is the expectation that a role will either be entirely work from home or at least offer flexibility, with some days being in the office and others being worked remotely. These expectations are taking hold across all industries. Employers who were initially forced to allow remote work found that productivity didn’t fall off. And many employees discovered they preferred working remotely — so much so, in fact, that they are determined to never return to an office environment.
How does this affect pharma companies’ quality departments? Some quality activities are difficult, but not impossible to perform remotely (e.g., inspections or audits), while others are very conducive to remote work if you have the right tools. For example, with a cloud based document management system, quality professionals can create, collaborate on, and gain approval for new standard operating procedures (SOPs) without setting foot in the office.
When pharma companies use AI-enabled technologies, they make the quality department more effective regardless of where they’re working from. With AI adoption increasing, it’s important to note that the demand for quality professionals isn’t going anywhere. AI will take on some of the time-consuming work that quality does now, but it will mostly serve to augment quality professionals and enable rapid decision-making.
The study from Cognizant makes this obvious. One of the questions asked of life sciences executives was to estimate what percentage of work would be done by machines. They were asked about eight categories, but interestingly in every case the executives expect less than 25% of the work to be done by intelligent machines. 24 The emphasis is increasingly on human-machine collaboration and getting the best of both worlds by letting the two complement each other. Pharma quality professionals won’t be losing their jobs to intelligent machines. Rather, modern tools will streamline processes and connect and contextualize data so human experts can focus on what they do best — ensuring that only the highest quality pharmaceutical products make it to market.
Summary
Quality isn’t exclusive to a single department. It’s all-pervasive and integral to every function throughout the enterprise.
Despite the inherent challenges of connecting quality and compliance data in an industry defined by truncated timelines, greater product complexity and personalization, and expanding supply chains and contract partner ecosystems, there are tools that can help you ensure quality’s connectedness and central bearing.
Connected applications, advanced analytics, and AI are becoming essential tools as the focus on data and predictive insights continues to mature in the pharmaceutical industry. They are empowering companies to simplify the adoption of a product life cycle approach to quality by converging data and processes within a centralized system that provides true quality intelligence and enables real-time decision-making.
MasterControl is the foundation for connected quality data and complete product quality. It unifies applications, data, and documentation across your entire product development life cycle, from concept to commercialization. MasterControl enables organizations like yours to go beyond proactive quality management and unleash the intelligence and insights hidden in your unstructured data.
Discover how MasterControl can make your vision of truly connected pharmaceutical quality data a reality. Contact a MasterControl representative today.