
3 Vital Steps to QMS Implementation
When shopping around for a quality management system (QMS), it might seem like you’ve tackled the biggest hurdle when you decide to purchase. According to MasterControl research, that isn’t necessarily the case. We found that while 85% of life sciences companies have purchased a QMS, the numbers on implementation are much lower.
In fact, only 29% have their QMS fully implemented at all facilities. We spoke with 152 life sciences quality professionals about digital transformation in their organisation and what effect it’s having in the quality department. The results showed that even though most respondents recognised the benefits of digitising, actually doing anything about it was a bit more difficult. That doesn’t mean it’s impossible. With proper planning, you can digitise and maximise your ROI.
Most companies use digital systems for quality management, but 32% of respondents in the study said they have significant issues associated with manual and paper-based processes. This is partly due to few processes being fully digital. For example, quality event management is fully digital at only 57% of companies and the numbers for other quality processes are even lower. This suggests that when a QMS is purchased, it isn’t fully implemented and/or used by most sites. To find out why, we looked into the blockers that hold companies back from initial purchase or expansion.
The biggest reasons companies don’t invest in or expand the use of their digital systems is, not surprisingly, money. For 46% of respondents, the cost of a new system couldn’t be justified. For another 39%, the time to transition and train employees was prohibitive. And in third place at 33% was that a new tool or system would disrupt business. These are legitimate concerns, but they’re ones that can be addressed by taking the right approach to implementation. When considering a QMS, your needs will only be met if you take the following steps.
1. Aligning People and Resources
The question of which QMS to purchase isn’t typically left up to a single person or department. Multiple people from multiple departments come together to make the decision. While that might seem to complicate things, in the long run this is actually good. Software adoption is challenging, but if you have all stakeholders that will sign off on the decision involved from the get-go, implementation and adoption will be much smoother.
To-Do List:
Identify key decision-makers.
Identify other executives whose support you need.
Set goals and expectations for time and involvement.
There are a couple of ways to win over other decision-makers. One of these is to focus on the benefits that come with increased digitisation. For example, most life sciences companies (58%) are driven to adopt digital solutions because it’s a competitive advantage in their industry. That’s a general benefit, but depending on the vendor you’re considering, there are more specific ones that can help. This is where it’s helpful to look at the functionality of the QMS and determine what’s important to other stakeholders. For example, a QMS that connects document, training, quality event, and audit management is going to be easier to adopt and more economical than shopping for all those systems separately.
Once everyone’s on board with the system, you can determine the resources you’ll need. This is when you’ll need to set goals and a timeline for implementation. It’s important to set expectations for those who will be most heavily involved in implementation and use of the new system. This will increase end user adoption and help them prepare for adjustments to their workloads to accommodate the time needed to adjust to the QMS.
2. Preparing for Configuration
When evaluating QMS vendors, one of the things to consider is how much assistance the vendor will give you when it comes to configuration. You shouldn’t be left to figure out everything for yourself. A prime example of this is software validation. The cautious approach to validation can take months to complete, but most of that work isn’t required by regulations. The U.S. Food and Drug Administration (FDA) and other regulators encourage companies to take a risk-based approach so they can focus their efforts on the areas that need it the most. To help our customers lighten the validation burden, MasterControl has the patented Validation Excellence Tool (VxT). Customers that use VxT for their initial validation can complete the process in approximately 20 hours and change control for an upgrade takes an average of 45 minutes. This greatly reduces the burden and friction that accompanies a more traditional validation approach.
Any time a business begins using a new system, getting data into that system takes time and the resulting disruption is one of the things that holds companies back. That’s why MasterControl created RapidOnboarder. RapidOnboarder automates aggregation and loading information into MasterControl, cutting down the time it takes to onboard.
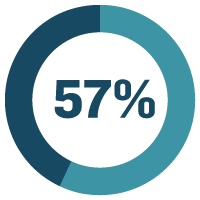
On average, MasterControl's RapidOnboarder tool reduces onboarding time by 57%.
Once you decide on a system, you have to get your end users to adopt it. That’s a whole other challenge and one that your vendor shouldn’t leave you to figure out yourself. Preparing work instructions for employees that will use the software is time consuming and assigning or changing roles is usually a one-by-one process. MasterControl’s Operations Procedure Builder (OPB) eliminates or lessens these problems. OPB automatically creates procedures and work instructions to reduce the workload associated with go-live. It also automatically assigns roles based on workflow data and lets you relabel roles all at once rather than doing it one at a time. MasterControl customers report that OPB takes processes that used to take weeks or months and lets them be done in hours or minutes.
Documents are a huge part of any QMS, so mapping your process for managing them is a must in configuration. You also need to identify who will have which rights in the system. In addition to documents, training and quality event management (QEM) are essential parts of your QMS that are all interconnected. Configuration is when you lay the groundwork for automation that will save you time later on. For example, if a quality event results in an updated standard operating procedure (SOP), you need some way to ensure that it does get updated, that anyone who needs to be trained on the changes is trained, and that the training gets recorded.
Map Your Document Process
Reviews
Approvals
Initiation
Revision
Collaboration
Archive
Who Will Have Which Rights
Creating
Using
Reviewing
Approving
Managing
MasterControl connects these processes for you. Part of your configuration is making these connections in the system so everything is triggered automatically. If your vendor can’t provide this level of automation and connectivity, you need to change your processes accordingly to ensure nothing falls through the cracks.
3. Go-Live and Training Employees
If you’ve properly prepared by following the previous steps, this one will be much easier. Every employee that will use the QMS needs training, although the extent of the training varies depending on how often they’ll use the QMS. One of the difficulties here is that people are resistant to change, even if it will ultimately help them. That’s why communication is so important leading up to go-live and why preparation needs to involve those who will use the system most. Without employee adoption, your company will never realise the full ROI potential of a QMS, and you’ll have resistance to further expansion to other sites.
Things To Consider
Who is responsible for training?
Who needs to be trained?
How will you prepare training materials?
How will you verify training?
What training needs to be recurring?
Of the blockers mentioned above, the ones that apply the most to end users are the time it will take to train on the new system and disruption to normal operations. Both of these are valid, but completely ignore the benefits that come from a digital QMS. You’ll save so much time and reduce overall disruption by bringing in a new system. That is the only way to make progress. You can minimise disruption by focusing on an efficient and effective training programme. This is an area where your software vendor can certainly help. They employ people whose job revolves around training others on the software. You can benefit from their experience by using their training best practices. At MasterControl, you have the option to be trained on our campus or to have one of our experts come to you.
Conclusion
Implementing a QMS does take time and effort, but a QMS is the gift that keeps on giving. It can put you into an audit-ready state, ensuring audits and inspections run well and greatly lowering your job-related stress. It automates many of the manual processes that consume a quality manager’s time, such as tracking down stakeholders to get signatures on documents, tracking training, and ensuring quality events are fully investigated and resolved. The benefits expand with your use of the system. With all sites using the same system, you can standardise quality processes across the board and increase visibility for everyone in the company.
One MasterControl customer, Fagron, has seen this as they’ve incrementally rolled out MasterControl to more of their worldwide locations. Fagron North America Quality Specialist Matt Seitz-Paquette commented, “We’re all working Industry from one source of truth on one platform. So, everybody around the world is together. Now I can see the documents that Poland is working on and vice versa. We haven’t had that flexibility until now.”
This increased flexibility and the time and money saved from using a digital system far surpasses the upfront cost of a QMS, time lost to training, and brief business disruption. Ultimately, this means you can get your life-changing products to more people sooner. Change is inevitable, one way or another. Business as usual can never be completely stagnant. Proactively deciding to change rather than waiting until you’re forced to change is always the better approach. Otherwise, you end up waiting until you receive a Form 483 from the FDA, or you’re forced to recall a product, or you face any other quality- or regulatory-related nightmare.
A lot of work goes into QMS implementation, and the work doesn’t end at go-live. Continual improvement requires continual effort. That’s why we’ll work with you through the process and continue to support you after implementation. By choosing a vendor that’s also a partner, you can ensure success in implementation and beyond.
Contact us today to learn how we can help your organisation accelerate implementation and start realizing the benefits of your QMS sooner.