
Avoid These Top 5
Quality Management Failures
Quality is something everyone takes for granted until something goes wrong. While the quality department is dedicated to quality, without proper support and resources from upper management and other departments, quality’s job is much harder. These are common problems that every quality professional faces. That doesn’t mean they’re unavoidable, though. In fact, with the right tools, you can avoid the very issues that cause others to fail.
To help you address and avoid these failures, we first needed to know which ones are the most common. So, we surveyed 152 life sciences quality professionals and asked about how significant certain problems that can lead to failure are in their organisations. The results are in the graph below. If you address these five issues, you can turn them from potential failures into differentiators that give you a competitive advantage and help you in your day-to-day work.
Top 5 Quality Management Pain Points

1. Manual/Paper-Based Processes
The biggest pain that’s causing failure in life sciences quality is paper and manual processes. Anyone who prints out standard operating procedures (SOPs) and stores them in binders or filing cabinets is already very familiar with these processes. Document control is vital to quality management and can include SOPs, training documents, work instructions, etc. When these are physically printed out and stored, that takes up space and costs money. It isn’t just the cost of printing — it’s also the cost of an off-site facility to store all your documents. And the cost to retrieve them. Version control is a problem when you can’t track down every printed copy and ensure it’s replaced with a newer version. And that doesn’t even take into account the hassle of collaborating on and approving a document that’s physically been printed out
These are problems in any manual quality process, even if physical paper isn’t involved. For example, you might use a spreadsheet to track who’s been trained on what. That still involves you physically going in and updating it. And it probably involves you sending out a reminder to employees to complete their training (possibly several reminders). Even documents saved on a computer present the same trouble with version control as paper documents. Granted, storage isn’t as big of an issue, but finding and retrieving a document can be equally as burdensome.
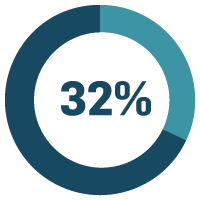
of life sciences companies say manual/paper-based processes are a major problem.
MasterControl Quality Excellence is an electronic quality management system (eQMS) that solves these problems. The solution is 21 CFR Part 11 compliant and lets you collaborate online and gather electronic signatures. You can also ensure that users only access the most recent version of a document because old versions will be automatically archived. Training is automated as well and can be connected to documents so that a new version of an SOP automatically sends out training tasks. A matrix in the system keeps track of training tasks that employees have completed so you no longer need to create and maintain a spreadsheet.
2. Time-Consuming Quality Administration
A quarter of life sciences quality professionals found quality administration to take too much time. Ideally, an eQMS should make your job easier, but with some systems, any time saved from the eQMS just gets used validating the software, turning a potential win into a failure. This can be a months-long process in some organisations, but it doesn’t have to be. Lengthy software validation is a result of companies wanting to stay compliant and as a result they tend to go the extra mile. But that’s all it is — extra. Validating every aspect of software as if it’s high risk doesn’t serve a purpose. It just creates a lot of documentation and takes a long time. A long validation process can make upgrading seem like an insurmountable task. With a new version of software being released every quarter, it doesn’t take long to fall very far behind.
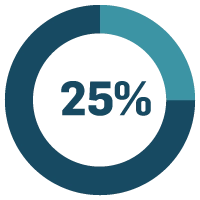
of life sciences companies say time-consuming quality administration is a major problem.
Validation can be performed much faster with a combination of the right tools and methodology. A risk-based approach is the one recommended by the U.S. Food and Drug Administration (FDA) and cuts down on the time needed. This limits extensive testing to functionality that is crucial to your business or to product safety and quality. A focus on truly high-risk functionality is useful to the company and proves compliance in a fraction of the time. This least-burdensome approach puts businesses in the perfect position to take advantage of frequent software upgrades so they can enjoy the latest enhancements. Anyone can use this approach on any software, but it’s so much easier if your vendor works with you to provide validation tools.
This is what MasterControl has done with our patented Validation Excellence Tool (VxT). VxT helps you determine which functionality presents the highest risk to your business. Using this tool, new customers can validate their MasterControl instance in 20 hours on average. When it comes to upgrades, our customers average only 45 minutes for software validation. And this is a tool that regularly comes under scrutiny during regulatory inspections. In fact, no customer has ever received a regulatory finding associated with VxT. We’re working to make validation an even faster process for our customers with our Validation on Demand (VoD) offering that lets users validate at any time with just a few clicks.
3. Managing Audits and Inspections
Part of that time-consuming quality administration can also be audits and inspections. This is an area of quality that you definitely don’t want to fail, but 24% of our respondents have significant pain associated with audits and inspections. Trying to schedule all audits and still be prepared at any time for a surprise inspection is difficult without a comprehensive eQMS. Then there’s the process of getting through the audit. Low-hanging fruit for an auditor includes documents and training records. If you’re dealing with paper, many of the pain points mentioned in the first section apply. However, this is a higher-risk scenario because it can result in losing a customer or certification, or receiving a Form 483 from the FDA.
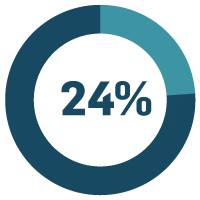
of life sciences companies say managing audits and inspections is a major problem.
The other factor to consider is that audits and inspections have changed considerably due to the COVID-19 pandemic. Some companies conducted remote audits before COVID, but the trend picked up considerably during the pandemic. Even regulators have started doing some things remotely that they used to do only in person as part of an inspection. Requesting records remotely is common to both audits and inspections now. For companies that use a paper-based document management system, this involves a lot of scanning. For companies that use an eQMS, this is a much easier process of compiling already electronic documents to share with the auditor.
MasterControl Quality Excellence connects audit, training, and document management. While this helps you pull documents and training records during the audit, it also helps with the aftermath. The audit or inspection itself is just part of the equation. Remediation is equally important. If an audit or inspection finding warrants corrective action, Quality Excellence ensures the action is taken and not just recorded on paper. All records associated with the audit, the corrective action/preventive action (CAPA), ensuing changes to processes, and any necessary training are connected.
4. Cost of Quality
One way or another, quality costs. The total cost of quality is the cost of both poor quality and good quality. ASQ estimates the cost of poor quality in a thriving company to be around 10%-15% of operations. That’s a very costly failure. This is most problematic when a company’s leaders don’t want to invest in quality. This approach leads to very little money spent on quality until something goes wrong. That could be in the form of a recall, excessive waste, repairs, etc. Two ways to prevent cost of poor quality is through quality assurance and training efforts. These also require a cost but save organisations money in the long run. It’s the long run part that keeps some executives from making the investment.
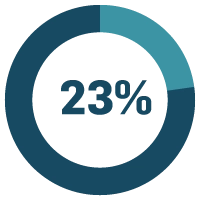
of life sciences companies say cost of quality is a major problem.
Unfortunately, it sometimes takes a major failure, like a recall, for companies to decide it is in fact worth it to invest in quality from the get-go. Neglecting quality has costs beyond the money required to make something right. There’s the negative press that comes with a quality problem. Quality problems can also be compliance problems, leading to product approval being delayed — sometimes indefinitely. On the other hand, investing in quality upfront can accelerate approval and improve the organisation’s reputation both with regulators and consumers.
MasterControl helps you reduce your cost of poor quality by giving you automated tools to assist your quality assurance efforts. Our eQMS helps you manage documents and training, but also helps you deal with quality events and suppliers. One of the best ways to reduce cost of poor quality is to extend your investment to the manufacturing floor. When you do this, you can make it impossible for an employee to fill out a production record if they haven’t been trained on the step. You can also get product out the door in a matter of hours by using review by exception.
5. Limited Quality System/Process Data Visibility
How much of your time is spent compiling data and preparing reports? This was a major pain for respondents in our study. Exporting data into a spreadsheet isn’t the best use of a quality manager’s time, but in some companies it’s the only way to gather data. The problem here isn’t just that it takes a lot of time, but also that manually reentering the data can introduce errors and the data is no longer up to date by the time it’s being used.
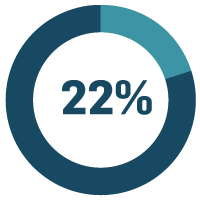
of life sciences companies say limited quality system/process data visibility is a major problem.
Relying on old data to make decisions defeats the whole point of using data in the first place. Even basic data analysis is a major accomplishment in such organisations. Trying to use artificial intelligence (AI) to improve operations would be out of the question.
Accurate data that’s available at a moment’s notice can change how a company operates. You can make decisions based on the latest data and have a reliable way to consistently measure if key performance indicators (KPIs) are improving. You’ll save time because you’ll no longer have to prepare reports for others. Instead of asking quality for a report, supervisors and managers can access the information themselves whenever they want it — and that’s way better than once a quarter.
MasterControl Insights provides that level of visibility in near real time. With Insights, you can easily see your data in prebuilt dashboards or design your own reports. While analytics and reporting are important for quality and compliance, the biggest potential lies in AI. Insights provides that AI functionality so you don’t have to go through the trouble of creating a program yourself. With our AI dashboards, you’ll know who’s going to be late on training before they’re late, and with natural language processing (NLP) we can tell you why documents tend to be rejected based on free text fields.
Conclusion
There’s no need to fail at quality management. These might be common issues that life sciences companies face, but that doesn’t mean they’re unavoidable. With the right tools, you can take these potential failures and turn them into major wins. MasterControl can help you do that. We’ve been providing quality management solutions to the life sciences industry for 30 years now. Our customers get rid of paper and manual processes, enjoy a lower overall cost of quality, have an easier time passing audits and inspections, have accessible data, and can validate their software much faster than any other eQMS.
Waiting to act is the most detrimental thing for your quality department. The longer you wait to make the move to digital solutions, the wider the gap gets between where your organization is and what’s possible to achieve in quality. Meanwhile, your competitors are moving to increase their investments in technology to get rid of these common quality pains. If the idea of making improvements is overwhelming, that’s all the more reason to begin now. We’re happy to help you start your journey and determine how to make incremental improvements to advance your company. Contact MasterControl today to see how we can help you avoid these failures and get your products to market faster.

