Your Guide to
FDA Product
Recall Avoidance
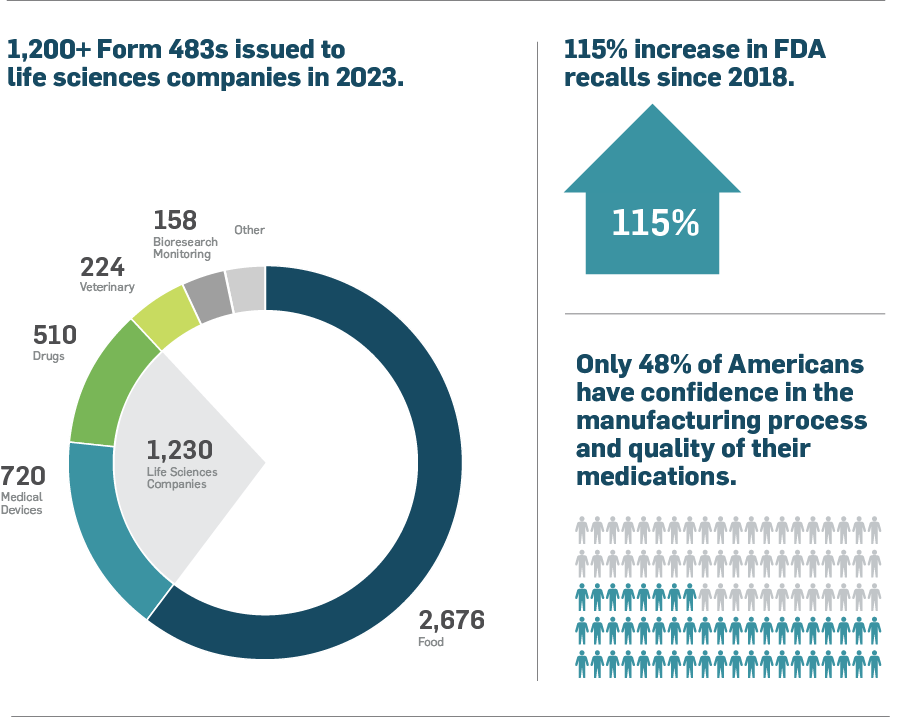
When life sciences companies fail to address the observations FDA inspectors identify in Form 483s, it can escalate to serious consequences like product recalls. Product recalls can be catastrophic, and recent trends are alarming.

1
Have effective quality event management procedures in place
With advanced, connected quality event management (QEM) software from MasterControl, you can easily maintain control of workflows, forms, steps, and data captures. MasterControl’s solution provides your teams with no-code workflow builders and customisable event templates that allow for simple and effective investigation, management, tracking, and reporting of quality events.
Learn about: MasterControl Advanced Quality Event Management (QEM)
2
Establish and document written procedures
Proven document management software lets you create and effectively track documents and their versions and revisions that can easily get lost or overlooked when on paper. MasterControl’s modern, connected system allows you to maintain all documents with time-stamped audit trails and 21 CFR Part 11-compliant signatures in a central, searchable location where they can be easily accessed by authorized personnel and auditors. Advanced document management capabilities even enable you to dynamically link standard operating procedures (SOPs) to batch records, ensuring your operators are following the most up-to-date procedures.
Learn about: MasterControl Documents
3
Properly investigate batch failures
Automating the management of quality control and quality assurance events and their resolution provides peace of mind that failure investigations are comprehensive. With a purpose-built, connected solution, you can leverage built-in controls and alerts to eliminate production errors. With MasterControl, our fully connected electronic batch records (EBR) and electronic device history records (eDHR) allow you to digitalise work instructions, launch quality events, and embed operator guidance and training. Plus, you can dramatically accelerate product reviews and releases if the solution includes real-time review-by-exception capabilities.
Learn about: MasterControl EBR/eDHR
4
Ensure adequate process validation
Easily generate, collaborate on, and maintain clear protocols for handling deviations from standard processes, ensuring thorough investigation of batch failures, and verifying process validation to prevent inconsistencies with MasterControl. MasterControl’s patented Validation Excellence Tool (VxT) can automatically produce the required validation documentation and identify additional testing needs with the click of a button, taking into account how your company uses the software and how your critical business processes are affected while providing the risk-based approach to validation recommended by the FDA.
Learn about: MasterControl Validation
5
Establish an effective training program and properly document procedures
Automate all your organisation’s training tasks, including records management, routing, tracking, follow-up, and escalation with MasterControl training management. This ensures that every employee’s training requirements are complete and up to date. And with training tasks embedded in fully connected EBRs/eDHRs, operator compliance is ensured.
Learn about: MasterControl Training
The MasterControl Platform
MasterControl’s fully integrated quality and manufacturing solutions are specifically designed to facilitate compliance and help companies avoid problems like product recalls. For more than three decades, life sciences companies have relied on MasterControl solutions to meet regulatory expectations and enjoy smoother, observation-free regulatory inspections.
Contact us today to find out how our purpose-built software solutions can help you protect consumers, preserve your standing in the market, and transform the challenge of compliance into a cornerstone of operational success.
Ready to Get Started?
Speak with one of the expert consultants at MasterControl to know how.